Molecular and Clinical Spectrum of Primary Hyperparathyroidism
- PMID: 36961765
- PMCID: PMC10502601
- DOI: 10.1210/endrev/bnad009
Molecular and Clinical Spectrum of Primary Hyperparathyroidism
Abstract
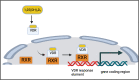
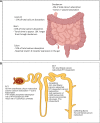
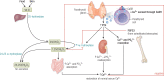
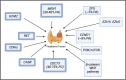
Recent data suggest an increase in the overall incidence of parathyroid disorders, with primary hyperparathyroidism (PHPT) being the most prevalent parathyroid disorder. PHPT is associated with morbidities (fractures, kidney stones, chronic kidney disease) and increased risk of death. The symptoms of PHPT can be nonspecific, potentially delaying the diagnosis. Approximately 15% of patients with PHPT have an underlying heritable form of PHPT that may be associated with extraparathyroidal manifestations, requiring active surveillance for these manifestations as seen in multiple endocrine neoplasia type 1 and 2A. Genetic testing for heritable forms should be offered to patients with multiglandular disease, recurrent PHPT, young onset PHPT (age ≤40 years), and those with a family history of parathyroid tumors. However, the underlying genetic cause for the majority of patients with heritable forms of PHPT remains unknown. Distinction between sporadic and heritable forms of PHPT is useful in surgical planning for parathyroidectomy and has implications for the family. The genes currently known to be associated with heritable forms of PHPT account for approximately half of sporadic parathyroid tumors. But the genetic cause in approximately half of the sporadic parathyroid tumors remains unknown. Furthermore, there is no systemic therapy for parathyroid carcinoma, a rare but potentially fatal cause of PHPT. Improved understanding of the molecular characteristics of parathyroid tumors will allow us to identify biomarkers for diagnosis and novel targets for therapy.
Keywords: PTH; calcium; genetics of hyperparathyroidism; parathyroid adenomas; parathyroid cancer; parathyroid tumors.
Published by Oxford University Press on behalf of the Endocrine Society 2023.
Figures







References
-
- Collip J, Clark E. Further studies on the physiological action of a parathyroid hormone. J Biol Chem. 1925;64(2):485‐507.
-
- Niall HD, Keutmann H, Sauer R, et al. The amino acid sequence of bovine parathyroid hormone I. Hoppe Seylers Z Physiol Chem. 1970;351(12):1586‐1588. - PubMed
-
- Summers GW. Parathyroid update: a review of 220 cases. Ear Nose Throat J. 1996;75(7):434‐439. - PubMed
-
- Thompson NW, Eckhauser FE, Harness JK. The anatomy of primary hyperparathyroidism. Surgery. 1982;92(5):814‐821. - PubMed

