Interrogating Encapsulated Protein Structure within Metal-Organic Frameworks at Elevated Temperature
- PMID: 36961883
- PMCID: PMC10080685
- DOI: 10.1021/jacs.2c13525
Interrogating Encapsulated Protein Structure within Metal-Organic Frameworks at Elevated Temperature
Abstract
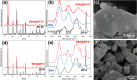
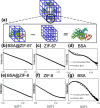
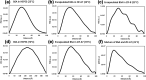
Encapsulating biomacromolecules within metal-organic frameworks (MOFs) can confer thermostability to entrapped guests. It has been hypothesized that the confinement of guest molecules within a rigid MOF scaffold results in heightened stability of the guests, but no direct evidence of this mechanism has been shown. Here, we present a novel analytical method using small-angle X-ray scattering (SAXS) to solve the structure of bovine serum albumin (BSA) while encapsulated within two zeolitic imidazolate frameworks (ZIF-67 and ZIF-8). Our approach comprises subtracting the scaled SAXS spectrum of the ZIF from that of the biocomposite BSA@ZIF to determine the radius of gyration of encapsulated BSA through Guinier, Kratky, and pair distance distribution function analyses. While native BSA exposed to 70 °C became denatured, in situ SAXS analysis showed that encapsulated BSA retained its size and folded state at 70 °C when encapsulated within a ZIF scaffold, suggesting that entrapment within MOF cavities inhibited protein unfolding and thus denaturation. This method of SAXS analysis not only provides insight into biomolecular stabilization in MOFs but may also offer a new approach to study the structure of other conformationally labile molecules in rigid matrices.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

