Structural basis of bacteriophage T5 infection trigger and E. coli cell wall perforation
- PMID: 36961893
- PMCID: PMC10038345
- DOI: 10.1126/sciadv.ade9674
Structural basis of bacteriophage T5 infection trigger and E. coli cell wall perforation
Abstract
Most bacteriophages present a tail allowing host recognition, cell wall perforation, and viral DNA channeling from the capsid to the infected bacterium cytoplasm. The majority of tailed phages bear a long flexible tail (Siphoviridae) at the tip of which receptor binding proteins (RBPs) specifically interact with their host, triggering infection. In siphophage T5, the unique RBP is located at the extremity of a central fiber. We present the structures of T5 tail tip, determined by cryo-electron microscopy before and after interaction with its E. coli receptor, FhuA, reconstituted into nanodisc. These structures bring out the important conformational changes undergone by T5 tail tip upon infection, which include bending of T5 central fiber on the side of the tail tip, tail anchoring to the membrane, tail tube opening, and formation of a transmembrane channel. The data allow to detail the first steps of an otherwise undescribed infection mechanism.
Figures

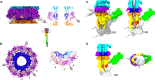



References
-
- C. A. Suttle, Marine viruses--major players in the global ecosystem. Nat. Rev. Microbiol. 5, 801–812 (2007). - PubMed
-
- S. Uyttebroek, B. Chen, J. Onsea, F. Ruythooren, Y. Debaveye, D. Devolder, I. Spriet, M. Depypere, J. Wagemans, R. Lavigne, J.-P. Pirnay, M. Merabishvili, P. De Munter, W. E. Peetermans, L. Dupont, L. Van Gerven, W.-J. Metsemakers, Safety and efficacy of phage therapy in difficult-to-treat infections: A systematic review. Lancet Infect. Dis. 22, e208–e220 (2022). - PubMed
-
- A. R. Davidson, L. Cardarelli, L. G. Pell, D. R. Radford, K. L. Maxwell, Long noncontractile tail machines of bacteriophages. Adv. Exp. Med. Biol. 726, 115–142 (2012). - PubMed
-
- M. Brackmann, S. Nazarov, J. Wang, M. Basler, Using force to punch holes: Mechanics of contractile nanomachines. Trends Cell Biol. 27, 623–632 (2017). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

