A stretchable wireless wearable bioelectronic system for multiplexed monitoring and combination treatment of infected chronic wounds
- PMID: 36961905
- PMCID: PMC10038347
- DOI: 10.1126/sciadv.adf7388
A stretchable wireless wearable bioelectronic system for multiplexed monitoring and combination treatment of infected chronic wounds
Abstract
Chronic nonhealing wounds are one of the major and rapidly growing clinical complications all over the world. Current therapies frequently require emergent surgical interventions, while abuse and misapplication of therapeutic drugs often lead to an increased morbidity and mortality rate. Here, we introduce a wearable bioelectronic system that wirelessly and continuously monitors the physiological conditions of the wound bed via a custom-developed multiplexed multimodal electrochemical biosensor array and performs noninvasive combination therapy through controlled anti-inflammatory antimicrobial treatment and electrically stimulated tissue regeneration. The wearable patch is fully biocompatible, mechanically flexible, stretchable, and can conformally adhere to the skin wound throughout the entire healing process. Real-time metabolic and inflammatory monitoring in a series of preclinical in vivo experiments showed high accuracy and electrochemical stability of the wearable patch for multiplexed spatial and temporal wound biomarker analysis. The combination therapy enabled substantially accelerated cutaneous chronic wound healing in a rodent model.
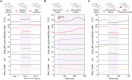
Figures






References
-
- G. C. Gurtner, S. Werner, Y. Barrandon, M. T. Longaker, Wound repair and regeneration. Nature 453, 314–321 (2008). - PubMed
-
- B. K. Sun, Z. Siprashvili, P. A. Khavari, Advances in skin grafting and treatment of cutaneous wounds. Science 346, 941–945 (2014). - PubMed
-
- C. E. Fife, M. J. Carter, Wound care outcomes and associated cost among patients treated in US outpatient wound centers: Data from the US wound registry. Wounds 24, 10–17 (2012). - PubMed
-
- D. G. Armstrong, A. J. M. Boulton, S. A. Bus, Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 376, 2367–2375 (2017). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

