Predicting Response of Triple-Negative Breast Cancer to Neoadjuvant Chemotherapy Using a Deep Convolutional Neural Network-Based Artificial Intelligence Tool
- PMID: 36961981
- PMCID: PMC10530970
- DOI: 10.1200/CCI.22.00181
Predicting Response of Triple-Negative Breast Cancer to Neoadjuvant Chemotherapy Using a Deep Convolutional Neural Network-Based Artificial Intelligence Tool
Abstract
Purpose: Achieving a pathological complete response (pCR) to neoadjuvant chemotherapy (NAC) is associated with improved patient outcomes in triple-negative breast cancer (TNBC). Currently, there are no validated predictive biomarkers for the response to NAC in TNBC. We developed and validated a deep convolutional neural network-based artificial intelligence (AI) model to predict the response of TNBC to NAC.
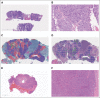
Materials and methods: Whole-slide images (WSIs) of hematoxylin and eosin-stained core biopsies from 165 (pCR in 60 and non-pCR in 105) and 78 (pCR in 31 and non-pCR in 47) patients with TNBC were used to train and validate the model. The model extracts morphometric features from WSIs in an unsupervised manner, thereby generating clusters of morphologically similar patterns. Downstream ranking of clusters provided regions of interest and morphometric scores; a low score close to zero and a high score close to one represented a high or low probability of response to NAC.
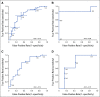
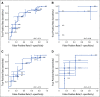
Results: The predictive ability of AI score for the entire cohort of 78 patients with TNBC ascertained by receiver operating characteristic analysis demonstrated an area under the curve (AUC) of 0.75. The AUC for stages I, II, and III disease were 0.88, 0.73, and 0.74, respectively. Using a cutoff value of 0.35, the positive predictive value of the AI score for pCR was 73.7%, and the negative predictive value was 76.2% for non-pCR patients.
Conclusion: To our knowledge, this study is the first to demonstrate the use of an AI tool on digitized hematoxylin and eosin-stained tissue images to predict the response to NAC in patients with TNBC with high accuracy. If validated in subsequent studies, these results may serve as an ancillary aid for individualized therapeutic decisions in patients with TNBC.
Conflict of interest statement
Figures

References
-
- Liedtke C, Mazouni C, Hess KR, et al. : Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26:1275-1281, 2008 - PubMed
-
- von Minckwitz G, Untch M, Blohmer JU, et al. : Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30:1796-1804, 2012 - PubMed
-
- Schmid P, Cortes J, Pusztai L, et al. : Pembrolizumabfor early triple-negative braest cancer. New Eng J Med 27:810-821, 2020 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

