Direct estimates of absolute ventilation and estimated Mycobacterium tuberculosis transmission risk in clinics in South Africa
- PMID: 36962521
- PMCID: PMC10021606
- DOI: 10.1371/journal.pgph.0000603
Direct estimates of absolute ventilation and estimated Mycobacterium tuberculosis transmission risk in clinics in South Africa
Abstract
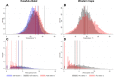
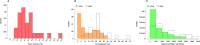
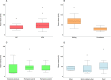
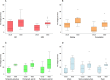
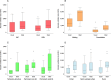
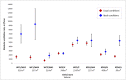
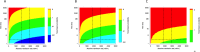
Healthcare facilities are important sites for the transmission of pathogens spread via bioaerosols, such as Mycobacterium tuberculosis. Natural ventilation can play an important role in reducing this transmission. We aimed to measure rates of natural ventilation in clinics in KwaZulu-Natal and Western Cape provinces, South Africa, then use these measurements to estimate Mycobacterium tuberculosis transmission risk. We measured ventilation in clinic spaces using a tracer-gas release method. In spaces where this was not possible, we estimated ventilation using data on indoor and outdoor carbon dioxide levels. Ventilation was measured i) under usual conditions and ii) with all windows and doors fully open. Under various assumptions about infectiousness and duration of exposure, measured absolute ventilation rates were related to risk of Mycobacterium tuberculosis transmission using the Wells-Riley Equation. In 2019, we obtained ventilation measurements in 33 clinical spaces in 10 clinics: 13 consultation rooms, 16 waiting areas and 4 other clinical spaces. Under usual conditions, the absolute ventilation rate was much higher in waiting rooms (median 1769 m3/hr, range 338-4815 m3/hr) than in consultation rooms (median 197 m3/hr, range 0-1451 m3/hr). When compared with usual conditions, fully opening existing doors and windows resulted in a median two-fold increase in ventilation. Using standard assumptions about infectiousness, we estimated that a health worker would have a 24.8% annual risk of becoming infected with Mycobacterium tuberculosis, and that a patient would have an 0.1% risk of becoming infected per visit. Opening existing doors and windows and rearranging patient pathways to preferentially use better ventilated clinic spaces result in important reductions in Mycobacterium tuberculosis transmission risk. However, unless combined with other tuberculosis infection prevention and control interventions, these changes are insufficient to reduce risk to health workers, and other highly exposed individuals, to acceptable levels.
Copyright: © 2022 Beckwith et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, et al.. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet (London, England). 2006;368: 1575–1580. doi: 10.1016/S0140-6736(06)69573-1 - DOI - PubMed
-
- Bantubani N, Kabera G, Connolly C, Rustomjee R, Reddy T, Cohen T, et al.. High rates of potentially infectious tuberculosis and multidrug-resistant tuberculosis (MDR-TB) among hospital inpatients in KwaZulu Natal, South Africa indicate risk of nosocomial transmission. PLoS One. 2014;9: 1–7. doi: 10.1371/journal.pone.0090868 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
