Target product profiles for novel medicines to prevent and treat preeclampsia: An expert consensus
- PMID: 36962694
- PMCID: PMC10021561
- DOI: 10.1371/journal.pgph.0001260
Target product profiles for novel medicines to prevent and treat preeclampsia: An expert consensus
Abstract
Background: Preeclampsia and eclampsia are a leading cause of global maternal and newborn mortality. Currently, there are few effective medicines that can prevent or treat preeclampsia. Target Product Profiles (TPPs) are important tools for driving new product development by specifying upfront the characteristics that new products should take. Considering the lack of investment and innovation around new medicines for obstetric conditions, we aimed to develop two new TPPs for medicines to prevent and treat preeclampsia.
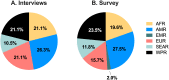
Methods and findings: We used a multi-methods approach comprised of a literature review, stakeholder interviews, online survey, and public consultation. Following an initial literature review, diverse stakeholders (clinical practice, research, academia, international organizations, funders, consumer representatives) were invited for in-depth interviews and an online international survey, as well as public consultation on draft TPPs. The level of stakeholder agreement with TPPs was assessed, and findings from interviews were synthesised to inform the final TPPs. We performed 23 stakeholder interviews and received 46 survey responses. A high level of agreement was observed in survey results, with 89% of TPP variables reaching consensus (75% agree or strongly agree). Points of discussion were raised around the target population for preeclampsia prevention and treatment, as well as the acceptability of cold-chain storage and routes of administration.
Conclusion: There is consensus within the maternal health research community on the parameters that new medicines for preeclampsia prevention and treatment must achieve to meet real-world health needs. These TPPs provide necessary guidance to spur interest, innovation and investment in the development of new medicines to prevent and treat preeclampsia.
Copyright: © 2022 Mcdougall et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Expert consensus on novel medicines to prevent preterm birth and manage preterm labour: Target product profiles.BJOG. 2024 Jan;131(1):71-80. doi: 10.1111/1471-0528.17314. Epub 2022 Oct 27. BJOG. 2024. PMID: 36209501
-
Target product profiles for neonatal care devices: systematic development and outcomes with NEST360 and UNICEF.BMC Pediatr. 2023 Nov 15;23(Suppl 2):564. doi: 10.1186/s12887-023-04342-1. BMC Pediatr. 2023. PMID: 37968603 Free PMC article.
-
Target Product Profile for a Machine Learning-Automated Retinal Imaging Analysis Software for Use in English Diabetic Eye Screening: Protocol for a Mixed Methods Study.JMIR Res Protoc. 2024 Mar 27;13:e50568. doi: 10.2196/50568. JMIR Res Protoc. 2024. PMID: 38536234 Free PMC article.
-
Facilitating the use of the target product profile in academic research: a systematic review.J Transl Med. 2024 Jul 29;22(1):693. doi: 10.1186/s12967-024-05476-1. J Transl Med. 2024. PMID: 39075460 Free PMC article.
-
Target Product Profiles for medical tests: a systematic review of current methods.BMC Med. 2020 May 11;18(1):119. doi: 10.1186/s12916-020-01582-1. BMC Med. 2020. PMID: 32389127 Free PMC article.
Cited by
-
Epigenetic biomarker for preeclampsia-associated preterm birth and potential preventative medicine.Environ Epigenet. 2024 Nov 6;10(1):dvae022. doi: 10.1093/eep/dvae022. eCollection 2024. Environ Epigenet. 2024. PMID: 39606093 Free PMC article.
-
Challenges in Conducting Clinical Trials for Preeclampsia.Curr Hypertens Rep. 2024 Feb;26(2):59-68. doi: 10.1007/s11906-023-01276-y. Epub 2023 Nov 16. Curr Hypertens Rep. 2024. PMID: 37971596 Review.
-
The drug drought in maternal health: an ongoing predicament.Lancet Glob Health. 2024 Jul;12(7):e1174-e1183. doi: 10.1016/S2214-109X(24)00144-X. Lancet Glob Health. 2024. PMID: 38876763 Free PMC article. Review.
-
Maternal gut microbiome interventions to improve maternal and perinatal health outcomes: Target product profile expert consensus and pipeline analysis.PLoS One. 2025 Jul 2;20(7):e0321543. doi: 10.1371/journal.pone.0321543. eCollection 2025. PLoS One. 2025. PMID: 40601677 Free PMC article.
References
LinkOut - more resources
Full Text Sources
