Seroprevalence of anti-SARS-CoV-2 antibodies among blood donors from December 2020 to June 2021 in Koutiala district, Mali
- PMID: 36962828
- PMCID: PMC10022217
- DOI: 10.1371/journal.pgph.0001316
Seroprevalence of anti-SARS-CoV-2 antibodies among blood donors from December 2020 to June 2021 in Koutiala district, Mali
Abstract
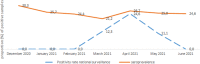
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the virus associated with coronavirus disease (COVID-19). At the time of the study, little data on the level of exposure of the population in Koutiala district in Mali to SARS-CoV-2 was available. Although blood donors are not representative of the general population, a COVID-19 seroprevalence estimate in this population was intended to assess the extent of community transmission, serve as a health alert system, and help guide the public health response. We measured seroprevalence of anti-SARS-CoV-2 antibodies using NG-Biotech SARS-Cov-2 RDT and ECLIA test between January and June 2020. This is a cross-sectional study of volunteer blood donors aged 18 to 60 years, independent of any previous COVID-19 disease. A stratified analysis of seroprevalence by month of sample collection and a comparison of the results of the NG-Biotech SARS-Cov-2 RDT with those of the ECLIA test was performed. The overall prevalence of antibodies to SARS-Cov-2 virus assessed by the NG-Biotech SARS-Cov-2 RDT was 24.6% (95% CI 21.8-27.4) and by the ECLIA test was 70.2 (95% CI 64.9-75.5). Both estimates remained relatively stable over the study period. We observed SARS-CoV-2 exposure much higher than indicated by case-based surveillance. The national surveillance system, as it was, was not able to detect variations in incidence, and as such, we do not recommend it as an alert system. However, the discrepancy between the results of the rapid test and the ECLIA test shows that further research is required to assess the validity of these test before a more solid conclusion can be drawn it their use in surveillance.
Copyright: © 2023 Temessadouno et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Johns Hopkins Coronavirus Resource Center. COVID-19 Map [Internet]. Available from: https://coronavirus.jhu.edu/map.html
-
- Slot E, Hogema BM, Reusken CBEM, Reimerink JH, Molier M, Karregat JHM, et al.. Herd immunity is not a realistic exit strategy during a COVID-19 outbreak [Internet]. In Review; 2020. Apr [cited 2022 May 25]. Available from: https://www.researchsquare.com/article/rs-25862/v1
-
- Thompson C. et al.. Neutralising antibodies to SARS coronavirus 2 in Scottish blood donors—a pilot study of the value of serology to determine population exposure. [Internet]. 2020. Available from: https://pesquisa.bvsalud.org/portal/resource/pt/ppmedrxiv-20060467?lang=en
LinkOut - more resources
Full Text Sources
Miscellaneous
