A multicenter survey of asymptomatic cryptococcal antigenemia among patients with advanced HIV disease in Nigeria
- PMID: 36963010
- PMCID: PMC10021610
- DOI: 10.1371/journal.pgph.0001313
A multicenter survey of asymptomatic cryptococcal antigenemia among patients with advanced HIV disease in Nigeria
Abstract
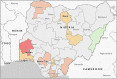
As of 2018, cryptococcal antigen (CrAg) screening in patients with advanced human immunodeficiency virus (HIV) disease (AHD) was not routinely implemented in Nigeria despite being recommended in the national HIV treatment guidelines. Our aim was to determine the prevalence and risk factors for asymptomatic cryptococcal antigenemia in adult people living with HIV (PLHIV) in Nigeria to advocate for the implementation of routine CrAg screening. A descriptive cross-sectional study and CrAg screening of consecutive adult PLHIV with CD4 counts ≤200 cells/μL was conducted from April 2018 to April 2019 at HIV clinics in eleven tertiary hospitals spread across Nigeria's six geopolitical regions. Prevalence of asymptomatic cryptococcal antigenemia was estimated by facility and geopolitical zone. Logistic regression was conducted to identify risk factors for cryptococcal antigenemia. In total, 1,114 patients with AHD were screened. The overall prevalence of asymptomatic cryptococcal antigenemia was 3.9% with wide variation across facilities (range: 0/75 [0%]- 15/122 [12.3%]) and geopolitical zones (range: 0/75 [0%]-19/279 [6.8%]). Prevalence of antigenemia was highest in the South-West (19/279 [6.8%]) and lowest in the North-East (0/75 [0%]). Prevalence was 5.2% (26/512) and 3.2% (18/561) in patients with CD4<100 and CD4 of 101-200, respectively. Of all patients with antigenemia, 50% were on antiretroviral therapy (ART) at the time of having a positive CrAg test. In adjusted analysis, cryptococcal antigenemia was significantly less in patients on ART and patients who had completed any formal education. The survey showed a high overall burden of cryptococcal antigenemia in Nigeria, with variable prevalence across geopolitical regions. We provided valuable evidence for implementing routine CrAg screening of AHD patients in Nigeria which has commenced in selected centres.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: DWD and family hold Founder shares in F2G Ltd, a University of Manchester spin-out antifungal discovery company, and share options in TFF Pharma. He acts or has recently acted as a consultant to Pulmatrix, Pulmocide, Biosergen, TFF Pharmaceuticals, Bright Angel Therapeutics and Cipla. He sits on the DSMB for a SARS CoV2 vaccine trial. In the last 3 years, he has been paid for talks on behalf of Hikma, Gilead, BioRad, Basilea, Mylan and Pfizer. DWD did not receive a salary from any of the commercial affiliations during the time of the study. Additionally, all commercial funding author DWD received were unrelated to, and outside of, the submitted work. All other authors declare that they have no financial or personal relationship(s) that may have inappropriately influenced them in writing this article. There are no patents, products in development or marketed products associated with this research to declare. The competing interests listed do not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- World Health Organization, 2017. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy, July 2017. - PubMed
-
- Tenforde M, Mokomane M, Leeme T, Patel R, Lekwape N, Ramodimoosi C, et al. Advanced human immunodeficiency virus disease in Botswana following successful antiretroviral therapy rollout: Incidence of and temporal trends in cryptococcal meningitis. Clin Infect Dis 2017; 65:779–786. doi: 10.1093/cid/cix430 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
