Clonal Characterization and Somatic Hypermutation Assessment by Next-Generation Sequencing in Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma: A Detailed Description of the Technical Performance, Clinical Utility, and Platform Comparison
- PMID: 36963483
- PMCID: PMC10243287
- DOI: 10.1016/j.jmoldx.2023.02.005
Clonal Characterization and Somatic Hypermutation Assessment by Next-Generation Sequencing in Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma: A Detailed Description of the Technical Performance, Clinical Utility, and Platform Comparison
Abstract
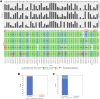
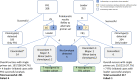
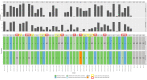
Somatic hypermutation status of the IGHV gene is essential for treating patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Unlike the conventional low-throughput method, assessment of somatic hypermutation by next-generation sequencing (NGS) has potential for uniformity and scalability. However, it lacks standardization or guidelines for routine clinical use. We critically assessed the performance of an amplicon-based NGS assay across 458 samples. Using a validation cohort (35 samples), the comparison of two platforms (Ion Torrent versus Illumina) and two primer sets [leader versus framework region 1 (FR1)] in their ability to identify clonotypic IGHV rearrangement(s) revealed 97% concordance. The mutation rates were identical by both platforms when using the same primer set (FR1), whereas a slight overestimation bias (+0.326%) was found when comparing FR1 with leader primers. However, for nearly all patients this did not affect the stratification into mutated or unmutated categories, suggesting that use of FR1 may provide comparable results if leader sequencing is not available and allowing for a simpler NGS laboratory workflow. In routine clinical practice (423 samples), the productive rearrangement was successfully detected by either primer set (leader, 97.7%; FR1, 94.7%), and a combination of both in problematic cases reduced the failure rate to 1.2%. Higher sensitivity of the NGS-based analysis also detected a higher frequency of double IGHV rearrangements (19.1%) compared with traditional approaches.
Copyright © 2023 Association for Molecular Pathology and American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Swerdlow S.H., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H., Thiele J., Arber D.A., Hasserjian R.P., Le Beau M.M., Orazi A., Siebert R. Lyon International Agency for Research on Cancer.; 2017. WHO Classification of Tumours of the Haematopoietic and Lymphoid Tissues.
-
- Hamblin T.J., Davis Z., Gardiner A., Oscier D.G., Stevenson F.K. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. - PubMed
-
- Damle R.N., Wasil T., Fais F., Ghiotto F., Valetto A., Allen S.L., Buchbinder A., Budman D., Dittmar K., Kolitz J., Lichtman S.M., Schulman P., Vinciguerra V.P., Rai K.R., Ferrarini M., Chiorazzi N. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–1847. - PubMed
-
- Parikh S.A., Strati P., Tsang M., West C.P., Shanafelt T.D. Should IGHV status and FISH testing be performed in all CLL patients at diagnosis? a systematic review and meta-analysis. Blood. 2016;127:1752–1760. - PubMed
-
- Thompson P.A., Tam C.S., O'Brien S.M., Wierda W.G., Stingo F., Plunkett W., Smith S.C., Kantarjian H.M., Freireich E.J., Keating M.J. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood. 2016;127:303–309. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

