Histopathology and SARS-CoV-2 Cellular Localization in Eye Tissues of COVID-19 Autopsies
- PMID: 36963628
- PMCID: PMC10032059
- DOI: 10.1016/j.ajpath.2023.02.016
Histopathology and SARS-CoV-2 Cellular Localization in Eye Tissues of COVID-19 Autopsies
Abstract
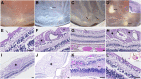
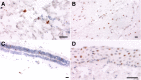
Ophthalmic manifestations and tissue tropism of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been reported in association with coronavirus disease 2019 (COVID-19), but the pathology and cellular localization of SARS-CoV-2 are not well characterized. The objective of this study was to evaluate macroscopic and microscopic changes and investigate cellular localization of SARS-CoV-2 across ocular tissues at autopsy. Ocular tissues were obtained from 25 patients with COVID-19 at autopsy. SARS-CoV-2 nucleocapsid gene RNA was previously quantified by droplet digital PCR from one eye. Herein, contralateral eyes from 21 patients were fixed in formalin and subject to histopathologic examination. Sections of the droplet digital PCR-positive eyes from four other patients were evaluated by in situ hybridization to determine the cellular localization of SARS-CoV-2 spike gene RNA. Histopathologic abnormalities, including cytoid bodies, vascular changes, and retinal edema, with minimal or no inflammation in ocular tissues were observed in all 21 cases evaluated. In situ hybridization localized SARS-CoV-2 RNA to neuronal cells of the retinal inner and outer layers, ganglion cells, corneal epithelia, scleral fibroblasts, and oligodendrocytes of the optic nerve. In conclusion, a range of common histopathologic alterations were identified within ocular tissue, and SARS-CoV-2 RNA was localized to multiple cell types. Further studies will be required to determine whether the alterations observed were caused by SARS-CoV-2 infection, the host immune response, and/or preexisting comorbidities.
Copyright © 2023 American Society for Investigative Pathology. All rights reserved.
Figures


References
-
- Stein S.R., Ramelli S.C., Grazioli A., Chung J.-Y., Singh M., Yinda C.K., Winkler C.W., Dickey J., Ylaya K., Ko S.H., Platt A., Burbelo P.D., Quezado M., Pittaluga S., Purcell M., Munster V.J., Belinky F., Ramos-Benitez M.J., Boritz E.A., Herr D.L., Rabin J., Saharia K.K., Madathil R.J., Tabatabai A., Soherwardi S., McCurdy M.T., NIH COVID-19 Autopsy Consortium, Peterson K.E., Cohen J.I., de Wit E., Vannella K.M., Hewitt S.M., Kleiner D.E., Chertow D.S. SARS-CoV-2 infection and persistence throughout the human body and brain. Nature. 2022;612:758–763. - PMC - PubMed
-
- Casagrande M., Fitzek A., Püschel K., Aleshcheva G., Schultheiss H.-P., Berneking L., Spitzer M.S., Schultheiss M. Detection of SARS-CoV-2 in human retinal biopsies of deceased COVID-19 patients. Ocul Immunol Inflamm. 2020;28:721–725. - PubMed
-
- Penkava J., Muenchhoff M., Badell I., Osterman A., Delbridge C., Niederbuchner F., Soliman S., Rudelius M., Graf A., Krebs S., Blum H., Ulbig M., Baumann C., Zapp D., Maier M., Keppler O.T., Lohmann C.P., Ledderose S. Detection of SARS-CoV-2-RNA in post-mortem samples of human eyes. Graefes Arch Clin Exp Ophthalmol. 2022;260:1789–1797. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

