Pathophysiology, emerging techniques for the assessment and novel treatment of aortic stenosis
- PMID: 36963766
- PMCID: PMC10040005
- DOI: 10.1136/openhrt-2022-002244
Pathophysiology, emerging techniques for the assessment and novel treatment of aortic stenosis
Abstract
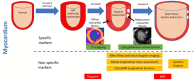
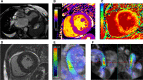
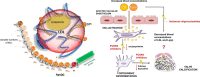
Our perspectives on aortic stenosis (AS) are changing. Evolving from the traditional thought of a passive degenerative disease, developing a greater understanding of the condition's mechanistic underpinning has shifted the paradigm to an active disease process. This advancement from the 'wear and tear' model is a result of the growing economic and health burden of AS, particularly within industrialised countries, prompting further research. The pathophysiology of calcific AS (CAS) is complex, yet can be characterised similarly to that of atherosclerosis. Progressive remodelling involves lipid-protein complexes, with lipoprotein(a) being of particular interest for diagnostics and potential future treatment options.There is an unmet clinical need for asymptomatic patient management; no pharmacotherapies are proven to slow progression and intervention timing varies. Novel approaches are developing to address this through: (1) screening with circulating biomarkers; (2) development of drugs to slow disease progression and (3) early valve intervention guided by medical imaging. Existing biomarkers (troponin and brain natriuretic peptide) are non-specific, but cost-effective predictors of ventricular dysfunction. In addition, their integration with cardiovascular MRI can provide accurate risk stratification, aiding aortic valve replacement decision making. Currently, invasive intervention is the only treatment for AS. In comparison, the development of lipoprotein(a) lowering therapies could provide an alternative; slowing progression of CAS, preventing left ventricular dysfunction and reducing reliance on surgical intervention.The landscape of AS management is rapidly evolving. This review outlines current understanding of the pathophysiology of AS, its management and future perspectives for the condition's assessment and treatment.
Keywords: AORTIC VALVE DISEASE; Aortic Valve Stenosis; Biomarkers; Diagnostic Imaging.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
