Complete bio-degradation of poly(butylene adipate-co-terephthalate) via engineered cutinases
- PMID: 36964144
- PMCID: PMC10039075
- DOI: 10.1038/s41467-023-37374-3
Complete bio-degradation of poly(butylene adipate-co-terephthalate) via engineered cutinases
Abstract
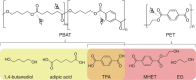
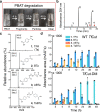
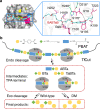
Poly(butylene adipate-co-terephthalate) (PBAT), a polyester made of terephthalic acid (TPA), 1,4-butanediol, and adipic acid, is extensively utilized in plastic production and has accumulated globally as environmental waste. Biodegradation is an attractive strategy to manage PBAT, but an effective PBAT-degrading enzyme is required. Here, we demonstrate that cutinases are highly potent enzymes that can completely decompose PBAT films in 48 h. We further show that the engineered cutinases, by applying a double mutation strategy to render a more flexible substrate-binding pocket exhibit higher decomposition rates. Notably, these variants produce TPA as a major end-product, which is beneficial feature for the future recycling economy. The crystal structures of wild type and double mutation of a cutinase from Thermobifida fusca in complex with a substrate analogue are also solved, elucidating their substrate-binding modes. These structural and biochemical analyses enable us to propose the mechanism of cutinase-mediated PBAT degradation.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Kawai F, Kawabata T, Oda M. Current state and perspectives related to the polyethylene terephthalate hydrolases available for biorecycling. ACS Sustain. Chem. Eng. 2020;8:8894–8908. doi: 10.1021/acssuschemeng.0c01638. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

