Type I interferon signaling in malignant blasts contributes to treatment efficacy in AML patients
- PMID: 36964168
- PMCID: PMC10039058
- DOI: 10.1038/s41419-023-05728-w
Type I interferon signaling in malignant blasts contributes to treatment efficacy in AML patients
Abstract
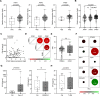
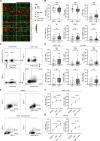
While type I interferon (IFN) is best known for its key role against viral infection, accumulating preclinical and clinical data indicate that robust type I IFN production in the tumor microenvironment promotes cancer immunosurveillance and contributes to the efficacy of various antineoplastic agents, notably immunogenic cell death inducers. Here, we report that malignant blasts from patients with acute myeloid leukemia (AML) release type I IFN via a Toll-like receptor 3 (TLR3)-dependent mechanism that is not driven by treatment. While in these patients the ability of type I IFN to stimulate anticancer immune responses was abolished by immunosuppressive mechanisms elicited by malignant blasts, type I IFN turned out to exert direct cytostatic, cytotoxic and chemosensitizing activity in primary AML blasts, leukemic stem cells from AML patients and AML xenograft models. Finally, a genetic signature of type I IFN signaling was found to have independent prognostic value on relapse-free survival and overall survival in a cohort of 132 AML patients. These findings delineate a clinically relevant, therapeutically actionable and prognostically informative mechanism through which type I IFN mediates beneficial effects in patients with AML.
© 2023. The Author(s).
Conflict of interest statement
LG is/has been holding research contracts with Lytix Biopharma, Promontory and Onxeo, has received consulting/advisory honoraria from Boehringer Ingelheim, AstraZeneca, OmniSEQ, Onxeo, The Longevity Labs, Inzen, Imvax, Sotio, Promontory, Noxopharm, EduCom, and the Luke Heller TECPR2 Foundation, and holds Promontory stock options. All other authors have no conflicts to declare.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

