Mesenchymal stem cell-derived extracellular vesicles subvert Th17 cells by destabilizing RORγt through posttranslational modification
- PMID: 36964252
- PMCID: PMC10073130
- DOI: 10.1038/s12276-023-00949-7
Mesenchymal stem cell-derived extracellular vesicles subvert Th17 cells by destabilizing RORγt through posttranslational modification
Erratum in
-
Author Correction: Mesenchymal stem cell-derived extracellular vesicles subvert Th17 cells by destabilizing RORγt through posttranslational modification.Exp Mol Med. 2023 Apr;55(4):870. doi: 10.1038/s12276-023-01001-4. Exp Mol Med. 2023. PMID: 37100897 Free PMC article. No abstract available.
Abstract
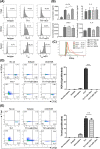
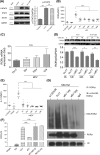
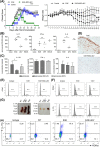
Mesenchymal stem cell (MSC)-derived small extracellular vesicles (MSC-sEVs) are known to exert immunosuppressive functions. This study showed that MSC-sEVs specifically convert T helper 17 (Th17) cells into IL-17 low-producer (ex-Th17) cells by degrading RAR-related orphan receptor γt (RORγt) at the protein level. In experimental autoimmune encephalomyelitis (EAE)-induced mice, treatment with MSC-sEVs was found to not only ameliorate clinical symptoms but also to reduce the number of Th17 cells in draining lymph nodes and the central nervous system. MSC-sEVs were found to destabilize RORγt by K63 deubiquitination and deacetylation, which was attributed to the EP300-interacting inhibitor of differentiation 3 (Eid3) contained in the MSC-sEVs. Small extracellular vesicles isolated from the Eid3 knockdown MSCs by Eid3-shRNA failed to downregulate RORγt. Moreover, forced expression of Eid3 by gene transfection was found to significantly decrease the protein level of RORγt in Th17 cells. Altogether, this study reveals the novel immunosuppressive mechanisms of MSC-sEVs, which suggests the feasibility of MSC-sEVs as an attractive therapeutic tool for curing Th17-mediated inflammatory diseases.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

