Study of FOXO1-interacting proteins using TurboID-based proximity labeling technology
- PMID: 36964488
- PMCID: PMC10039511
- DOI: 10.1186/s12864-023-09238-z
Study of FOXO1-interacting proteins using TurboID-based proximity labeling technology
Abstract
Background: Protein‒protein interactions (PPIs) are the foundation of the life activities of cells. TurboID is a biotin ligase with higher catalytic efficiency than BioID or APEX that reduces the required labeling time from 18 h to 10 min. Since many proteins participate in binding and catalytic events that are very short-lived, it is theoretically possible to find relatively novel binding proteins using the TurboID technique. Cell proliferation, apoptosis, autophagy, oxidative stress and metabolic disorders underlie many diseases, and forkhead box transcription factor 1 (FOXO1) plays a key role in these physiological and pathological processes.
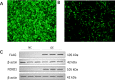
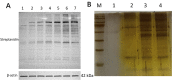
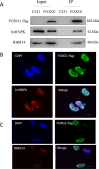
Results: The FOXO1-TurboID fusion gene was transfected into U251 astrocytes, and a cell line stably expressing FOXO1 was constructed. While constructing the FOXO1 overexpression plasmid, we also added the gene sequence of TurboID to perform biotin labeling experiments in the successfully fabricated cell line to look for FOXO1 reciprocal proteins. Label-free mass spectrometry analysis was performed, and 325 interacting proteins were found. A total of 176 proteins were identified in the FOXO1 overexpression group, and 227 proteins were identified in the Lipopolysaccharide -treated group (Lipopolysaccharide, LPS). Wild-type U251 cells were used to exclude interference from nonspecific binding. The FOXO1-interacting proteins hnRNPK and RBM14 were selected for immunoprecipitation and immunofluorescence verification.
Conclusion: The TurboID technique was used to select the FOXO1-interacting proteins, and after removing the proteins identified in the blank group, a large number of interacting proteins were found in both positive groups. This study lays a foundation for further study of the function of FOXO1 and the regulatory network in which it is involved.
Keywords: Biotin labeling; FOXO1; TurboID; U251 cells; hnRNPK.
© 2023. The Author(s).
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures


Similar articles
-
Study on the interaction protein of transcription factor Smad3 based on TurboID proximity labeling technology.Genomics. 2024 May;116(3):110839. doi: 10.1016/j.ygeno.2024.110839. Epub 2024 Mar 26. Genomics. 2024. PMID: 38537808
-
TurboID functions as an efficient biotin ligase for BioID applications in Xenopus embryos.Dev Biol. 2022 Dec;492:133-138. doi: 10.1016/j.ydbio.2022.10.005. Epub 2022 Oct 18. Dev Biol. 2022. PMID: 36270327 Free PMC article.
-
AirID, a novel proximity biotinylation enzyme, for analysis of protein-protein interactions.Elife. 2020 May 11;9:e54983. doi: 10.7554/eLife.54983. Elife. 2020. PMID: 32391793 Free PMC article.
-
The regulation of FOXO1 and its role in disease progression.Life Sci. 2018 Jan 15;193:124-131. doi: 10.1016/j.lfs.2017.11.030. Epub 2017 Nov 20. Life Sci. 2018. PMID: 29158051 Review.
-
Potential application of TurboID-based proximity labeling in studying the protein interaction network in plant response to abiotic stress.Front Plant Sci. 2022 Aug 16;13:974598. doi: 10.3389/fpls.2022.974598. eCollection 2022. Front Plant Sci. 2022. PMID: 36051300 Free PMC article. Review.
Cited by
-
Proteome profiling of cerebrospinal fluid using machine learning shows a unique protein signature associated with APOE4 genotype.Aging Cell. 2025 Apr;24(4):e14439. doi: 10.1111/acel.14439. Epub 2024 Dec 25. Aging Cell. 2025. PMID: 39722190 Free PMC article.
-
A Straightforward Interpretation of Proximity Labeling through Direct Biotinylation Analysis.ACS Omega. 2025 Jun 11;10(24):26098-26105. doi: 10.1021/acsomega.5c03099. eCollection 2025 Jun 24. ACS Omega. 2025. PMID: 40584372 Free PMC article.
-
Comprehensive dataset of interactors for the entire PARP family using TurboID proximity labeling.Sci Data. 2025 Mar 8;12(1):405. doi: 10.1038/s41597-025-04722-5. Sci Data. 2025. PMID: 40057523 Free PMC article.
-
APOE-NOTCH axis governs elastogenesis during human cardiac valve remodeling.Nat Cardiovasc Res. 2024 Aug;3(8):933-950. doi: 10.1038/s44161-024-00510-3. Epub 2024 Jul 24. Nat Cardiovasc Res. 2024. PMID: 39196035
-
Insights into Noncanonical and Diversified Functions of ABCF1: From Health to Disease.J Mol Biol. 2025 Sep 1;437(17):169286. doi: 10.1016/j.jmb.2025.169286. Epub 2025 Jun 11. J Mol Biol. 2025. PMID: 40513648 Review.
References
-
- Ko YS, Cho SJ, Park J, Kim Y, Choi YJ, Pyo JS, Jang BG, Park JW, Kim WH, Lee BL. Loss of FOXO1 promotes gastric tumour growth and metastasis through upregulation of human epidermal growth factor receptor 2/neu expression. Br J Cancer. 2015;113(8):1186–96. doi: 10.1038/bjc.2015.273. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

