Cerebral Aβ deposition precedes reduced cerebrospinal fluid and serum Aβ42/Aβ40 ratios in the AppNL-F/NL-F knock-in mouse model of Alzheimer's disease
- PMID: 36964585
- PMCID: PMC10039589
- DOI: 10.1186/s13195-023-01196-8
Cerebral Aβ deposition precedes reduced cerebrospinal fluid and serum Aβ42/Aβ40 ratios in the AppNL-F/NL-F knock-in mouse model of Alzheimer's disease
Abstract
Background: Aβ42/Aβ40 ratios in cerebrospinal fluid (CSF) and blood are reduced in preclinical Alzheimer's disease (AD), but their temporal and correlative relationship with cerebral Aβ pathology at this early disease stage is not well understood. In the present study, we aim to investigate such relationships using App knock-in mouse models of preclinical AD.
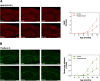
Methods: CSF, serum, and brain tissue were collected from 3- to 18-month-old AppNL-F/NL-F knock-in mice (n = 48) and 2-18-month-old AppNL/NL knock-in mice (n = 35). The concentrations of Aβ42 and Aβ40 in CSF and serum were measured using Single molecule array (Simoa) immunoassays. Cerebral Aβ plaque burden was assessed in brain tissue sections by immunohistochemistry and thioflavin S staining. Furthermore, the concentrations of Aβ42 in soluble and insoluble fractions prepared from cortical tissue homogenates were measured using an electrochemiluminescence immunoassay.
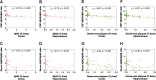
Results: In AppNL-F/NL-F knock-in mice, Aβ42/Aβ40 ratios in CSF and serum were significantly reduced from 12 and 16 months of age, respectively. The initial reduction of these biomarkers coincided with cerebral Aβ pathology, in which a more widespread Aβ plaque burden and increased levels of Aβ42 in the brain were observed from approximately 12 months of age. Accordingly, in the whole study population, Aβ42/Aβ40 ratios in CSF and serum showed a negative hyperbolic association with cerebral Aβ plaque burden as well as the levels of both soluble and insoluble Aβ42 in the brain. These associations tended to be stronger for the measures in CSF compared with serum. In contrast, no alterations in the investigated fluid biomarkers or apparent cerebral Aβ plaque pathology were found in AppNL/NL knock-in mice during the observation time.
Conclusions: Our findings suggest a temporal sequence of events in AppNL-F/NL-F knock-in mice, in which initial deposition of Aβ aggregates in the brain is followed by a decline of the Aβ42/Aβ40 ratio in CSF and serum once the cerebral Aβ pathology becomes significant. Our results also indicate that the investigated biomarkers were somewhat more strongly associated with measures of cerebral Aβ pathology when assessed in CSF compared with serum.
Keywords: Alzheimer’s disease; Beta-amyloid; Biomarker; Blood; Cerebrospinal fluid.
© 2023. The Author(s).
Conflict of interest statement
OH has acquired research support (for the institution) from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche. In the past 2 years, he has received consultancy/speaker fees from AC Immune, Amylyx, Alzpath, BioArctic, Biogen, Cerveau, Fujirebio, Genentech, Novartis, Roche, and Siemens.
HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Passage Bio, Pinteon Therapeutics, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work).
KB has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, BioArctic, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Ono Pharma, Pharmatrophix, Prothena, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper.
Figures


References
-
- Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43) Neuron. 1994;13(1):45–53. doi: 10.1016/0896-6273(94)90458-8. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

