Combining nanotechnology with monoclonal antibody drugs for rheumatoid arthritis treatments
- PMID: 36964609
- PMCID: PMC10039584
- DOI: 10.1186/s12951-023-01857-8
Combining nanotechnology with monoclonal antibody drugs for rheumatoid arthritis treatments
Abstract
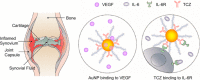
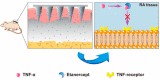
Rheumatoid arthritis (RA) is a systemic immune disease characterized by synovial inflammation. Patients with RA commonly experience significant damage to their hand and foot joints, which can lead to joint deformities and even disability. Traditional treatments have several clinical drawbacks, including unclear pharmacological mechanisms and serious side effects. However, the emergence of antibody drugs offers a promising approach to overcome these limitations by specifically targeting interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and other cytokines that are closely related to the onset of RA. This approach reduces the incidence of adverse effects and contributes to significant therapeutic outcomes. Furthermore, combining these antibody drugs with drug delivery nanosystems (DDSs) can improve their tissue accumulation and bioavailability.Herein, we provide a summary of the pathogenesis of RA, the available antibody drugs and DDSs that improve the efficacy of these drugs. However, several challenges need to be addressed in their clinical applications, including patient compliance, stability, immunogenicity, immunosupression, target and synergistic effects. We propose strategies to overcome these limitations. In summary, we are optimistic about the prospects of treating RA with antibody drugs, given their specific targeting mechanisms and the potential benefits of combining them with DDSs.
Keywords: Antibody drugs; Drug delivery nanosystem; Rheumatoid arthritis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

