S2k guidelines on the management of paraneoplastic pemphigus/paraneoplastic autoimmune multiorgan syndrome initiated by the European Academy of Dermatology and Venereology (EADV)
- PMID: 36965110
- PMCID: PMC10806824
- DOI: 10.1111/jdv.18931
S2k guidelines on the management of paraneoplastic pemphigus/paraneoplastic autoimmune multiorgan syndrome initiated by the European Academy of Dermatology and Venereology (EADV)
Erratum in
-
Corrigendum: S2k guidelines on the management of paraneoplastic pemphigus/paraneoplastic autoimmune multiorgan syndrome initiated by the European Academy of Dermatology and Venereology (EADV).J Eur Acad Dermatol Venereol. 2023 Nov;37(11):2378-2379. doi: 10.1111/jdv.19489. Epub 2023 Sep 13. J Eur Acad Dermatol Venereol. 2023. PMID: 37702229 No abstract available.
Abstract
Background: Paraneoplastic pemphigus (PNP), also called paraneoplastic autoimmune multiorgan syndrome (PAMS), is a rare autoimmune disease with mucocutaneous and multi-organ involvement. PNP/PAMS is typically associated with lymphoproliferative or haematological malignancies, and less frequently with solid malignancies. The mortality rate of PNP/PAMS is elevated owing to the increased risk of severe infections and disease-associated complications, such as bronchiolitis obliterans.
Objectives: These guidelines summarize evidence-based and expert-based recommendations (S2k level) for the clinical characterization, diagnosis and management of PNP/PAMS. They have been initiated by the Task Force Autoimmune Blistering Diseases of the European Academy of Dermatology and Venereology with the contribution of physicians from all relevant disciplines. The degree of consent among all task force members was included.
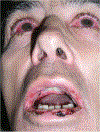
Results: Chronic severe mucositis and polymorphic skin lesions are clue clinical characteristics of PNP/PAMS. A complete assessment of the patient with suspected PNP/PAMS, requiring histopathological study and immunopathological investigations, including direct and indirect immunofluorescence, ELISA and, where available, immunoblotting/immunoprecipitation, is recommended to achieve a diagnosis of PNP/PAMS. Detection of anti-envoplakin antibodies and/or circulating antibodies binding to the rat bladder epithelium at indirect immunofluorescence is the most specific tool for the diagnosis of PNP/PAMS in a patient with compatible clinical and anamnestic features. Treatment of PNP/PAMS is highly challenging. Systemic steroids up to 1.5 mg/kg/day are recommended as first-line option. Rituximab is also recommended in patients with PNP/PAMS secondary to lymphoproliferative conditions but might also be considered in cases of PNP/PAMS associated with solid tumours. A multidisciplinary approach involving pneumologists, ophthalmologists and onco-haematologists is recommended for optimal management of the patients.
Conclusions: These are the first European guidelines for the diagnosis and management of PNP/PAMS. Diagnostic criteria and therapeutic recommendations will require further validation by prospective studies.
© 2023 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Conflict of interest statement
Emiliano Antiga, Rikke Bech, Barbara Bockle, Marzia Caproni, Nisha Suyen Chandran, Alberto Corrà, Francesco D’Amore, Maryam Daneshpazhooh, Dario Didona, Marian Dmochowski, Kossara Drenovska, Jan Ehrchen, Claudio Feliciani, Giovanni Genovese, Richard Groves, Sanjeev Handa, Takashi Hashimoto, Silke C. Hofmann, Dimitrios Ioannidis, Hana Jedlickova,Cezary Kowalewski, Khalaf Kridin, Roberto Maglie, Branka Marinovic, Angelo V. Marzano, Josè M.Mascarò Jr, Emanual Maverakis, Joost M. Meijer, Aikaterini Patsatsi, Catherine Prost, Jane Setterfield, Dusan Skiljevic, Eli Sprecher, Soner Uzun, Snejina Vassileva, Artem Vorobyev, Gang Wang, Mingyue Wang, Katarzyna Wozniak and Savas Yayli declared no conflict of interest.Luca Borradori had consulting fees from Chugai and Argenx; Frédéric Caux had grants with payment to his institution from Argenx, Regeneron and Roche; Dipankar De had a grant as an investigator from Argenx, Matthias Goebeler had a grant as an investigator with payment to his institution from Argenx, Claudia Gunther had a grant for attending a meeting by Boehringer; Barbara Horvath had grants with payment to her institutions from Janssen-Cilag, AbbVie, Novartis Pharma, Celgene/AMGEN, Akari therapeutics, Argenx, consulting fees with payment to her institution from Janssen-Cilag, AbbVie, Novartis Pharma, UCB Pharma, Leo Pharma, Akari therapeutics, Philips, Roche, Regeneron, Sanofi, Argenx, honoraria for lectures with payment to her institutions from Janssen-Cilag, AbbVie, Novartis Pharma, UCB Pharma, Leo Pharma, Solenne B.V., Celgene, Akari therapeutics, Philips, Roche, Regeneron, Sanofi, Argenx; Pascal Joly had consulting fees from Lilly, Novartis, Principia Biopharma, Roche Laboratories, Sanofi-aventis, Astra Zeneca, Argenx; Yen Loo Lim is in the Scientific programme committee and Organising committee of the World Congress of Dermatology 2023; Carlo Pincelli is consultant of Pincell and inventor in the patent:” Remedies for pemphigus containing anti Fas ligand antibodies” WO2010/066914; Enno Schmidt had grants with payment to his insititution from UCB, Incyte, Biotest, Argenx, Dompe, Euroimmun, Fresenius Medical Care, Bayer, Pharmaxis, Alpine Immune, CSL, had consulting fees from Leo, Chugai, Almirall, Janssen, had patents with Euroimmun and Dompe, had Participation on a Data Safety Monitoring Board or Advisory Board with Argenx and AstraZeneca; Kaisa Tasanen had support to attend EADV Congress from Novartis, participated to advisory board for Abbvie, Leo Pharma, Novartis, Sanofi Genzyme, Janssen-Cilag, Bristol-Myers Squibb and UCB Pharma and is Board member of ESDR and EDF; Nina Van Beek has a pending patent together with Euroimmun and received material for a joint project by Euroimmun; Igor Vujic had consulting fees from Abbvie, Novartis, Lilly, Amgen, Janssen; Giovanna Zambruno participated to advisory board with payment to her institution from Argenx.
Figures



References
-
- Anhalt GJ, Kim SC, Stanley JR, et al. Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia. N Engl J Med 1990;323:1729–35. - PubMed
-
- Anhalt GJ. Paraneoplastic pemphigus. Adv Dermatol 1997;12:77–96; discussion 97. - PubMed
-
- Nguyen VT, Ndoye A, Bassler KD, et al. Classification, clinical manifestations, and immunopathological mechanisms of the epithelial variant of paraneoplastic autoimmune multiorgan syndrome: a reappraisal of paraneoplastic pemphigus. Arch Dermatol 2001;137:193–206. - PubMed
-
- Schmidt E, Kasperkiewicz M, Joly P. Pemphigus. Lancet 2019;394:882–94. - PubMed

