The association between white matter hyperintensities and amyloid and tau deposition
- PMID: 36965457
- PMCID: PMC10060905
- DOI: 10.1016/j.nicl.2023.103383
The association between white matter hyperintensities and amyloid and tau deposition
Abstract
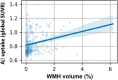
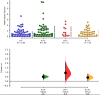
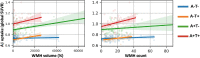
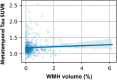
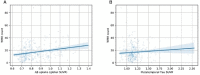
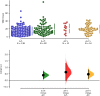
White matter hyperintensities (WMHs) frequently occur in Alzheimer's Disease (AD) and have a contribution from ischemia, though their relationship with β-amyloid and cardiovascular risk factors (CVRFs) is not completely understood. We used AT classification to categorize individuals based on their β-amyloid and tau pathologies, then assessed the effects of β-amyloid and tau on WMH volume and number. We then determined regions in which β-amyloid and WMH accumulation were related. Last, we analyzed the effects of various CVRFs on WMHs. As secondary analyses, we observed effects of age and sex differences, atrophy, cognitive scores, and APOE genotype. PET, MRI, FLAIR, demographic, and cardiovascular health data was collected from the Alzheimer's Disease Neuroimaging Initiative (ADNI-3) (N = 287, 48 % male). Participants were categorized as A + and T + if their Florbetapir SUVR and Flortaucipir SUVR were above 0.79 and 1.25, respectively. WMHs were mapped on MRI using a deep convolutional neural network (Sepehrband et al., 2020). CVRF scores were based on history of hypertension, systolic and diastolic blood pressure, pulse rate, respiration rate, BMI, and a cumulative score with 6 being the maximum score. Regression models and Pearson correlations were used to test associations and correlations between variables, respectively, with age, sex, years of education, and scanner manufacturer as covariates of no interest. WMH volume percent was significantly associated with global β-amyloid (r = 0.28, p < 0.001), but not tau (r = 0.05, p = 0.25). WMH volume percent was higher in individuals with either A + or T + pathology compared to controls, particularly within in the A+/T + group (p = 0.007, Cohen's d = 0.4, t = -2.5). Individual CVRFs nor cumulative CVRF scores were associated with increased WMH volume. Finally, the regions where β-amyloid and WMH count were most positively associated were the middle temporal region in the right hemisphere (r = 0.18, p = 0.002) and the fusiform region in the left hemisphere (r = 0.017, p = 0.005). β-amyloid and WMH have a clear association, though the mechanism facilitating this association is still not fully understood. The associations found between β-amyloid and WMH burden emphasizes the relationship between β-amyloid and vascular lesion formation while factors like CVRFs, age, and sex affect AD development through various mechanisms. These findings highlight potential causes and mechanisms of AD as targets for future preventions and treatments. Going forward, a larger emphasis may be placed on β-amyloid's vascular effects and the implications of impaired brain clearance in AD.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Avants, B., Tustison, N.J., Song, G., 2009. Advanced Normalization Tools: V1.0. 10.54294/uvnhin.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

