Fusion peptide is superior to co-expressing subunits for arming oncolytic herpes virus with interleukin 12
- PMID: 36966232
- PMCID: PMC10039936
- DOI: 10.1038/s43856-023-00270-4
Fusion peptide is superior to co-expressing subunits for arming oncolytic herpes virus with interleukin 12
Abstract
Background: G47∆ is a triple-mutated oncolytic herpes simplex virus type 1 (HSV-1) recently approved as a new drug for malignant glioma in Japan. As the next-generation, we develop armed oncolytic HSV-1 using G47∆ as the backbone. Because oncolytic HSV-1 elicits specific antitumor immunity, interleukin 12 (IL-12) can function as an effective payload to enhance the efficacy.
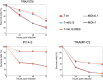
Methods: We evaluate the optimal methods for expressing IL-12 as a payload for G47∆-based oncolytic HSV-1. Two new armed viruses are generated for evaluation by employing different methods to express IL-12: T-mfIL12 expresses murine IL-12 as a fusion peptide, with the genes of two subunits (p35 and p40) linked by bovine elastin motifs, and T-mIL12-IRES co-expresses the subunits, with the two genes separated by an internal ribosome entry site (IRES) sequence.
Results: T-mfIL12 is significantly more efficient in producing IL-12 than T-mIL12-IRES in all cell lines tested, whereas the expression methods do not affect the replication capabilities and cytopathic effects. In two syngeneic mouse subcutaneous tumor models of Neuro2a and TRAMP-C2, T-mfIL12 exhibits a significantly higher efficacy than T-mIL12-IRES when inoculated intratumorally. Furthermore, T-mfIL12 shows a significantly higher intratumoral expression of functional IL-12, causing stronger stimulation of specific antitumor immune responses than T-mIL12-IRES.
Conclusions: The results implicate that a fusion-type expression of IL-12 is a method superior to co-expression of separate subunits, due to higher production of functional IL-12 molecules. This study led to the creation of triple-mutated oncolytic HSV-1 armed with human IL-12 currently used in phase 1/2 trial for malignant melanoma.
Plain language summary
Some viruses, including the herpes virus, can be modified so that they can target and kill cancers. These viruses can be loaded with factors that stimulate the immune system, which can help to eradicate cancer cells. Here, we test different methods of loading a cancer-killing version of the herpes virus with interleukin 12, an immune-stimulating factor. We show that one method, which involves loading the virus with the different parts of interleukin 12 fused together, is superior to another, and leads to improved anti-cancer effects in mouse models. These findings have contributed to the creation of a cancer-killing virus that is currently in clinical trials in patients with melanoma.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

