Time-dependent effect of GLP-1 receptor agonists on cardiovascular benefits: a real-world study
- PMID: 36966321
- PMCID: PMC10039680
- DOI: 10.1186/s12933-023-01800-z
Time-dependent effect of GLP-1 receptor agonists on cardiovascular benefits: a real-world study
Abstract
Background: Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have shown cardiovascular benefits in cardiovascular outcome trials in type 2 diabetes mellitus. However, the most convincing evidence was obtained in subjects with established cardiovascular (CV) disease. We analyzed the determinants of GLP-1 RA-mediated CV protection in a real-world population of persons with type 2 diabetes with and without a history of CV events with long-term follow-up.
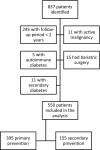
Methods: Retrospective cohort study of 550 individuals with type 2 diabetes (395 in primary CV prevention, 155 in secondary CV prevention), followed at a single center after the first prescription of a GLP-1 RA between 2009 and 2019. CV and metabolic outcomes were assessed.
Results: Median duration of follow-up was 5.0 years (0.25-10.8) in primary prevention and 3.6 years (0-10.3) in secondary prevention, with a median duration of treatment of 3.2 years (0-10.8) and 2.5 years (0-10.3) respectively. In the multivariable Cox regression model considering GLP-1 RA treatment as a time-dependent covariate, in the primary prevention group, changes in BMI and glycated hemoglobin did not have an impact on MACE risk, while age at the time of GLP-1 initiation (HR 1.08, 95% CI 1.03-1.14, p = 0.001) and GLP-1 RA cessation by time (HR 3.40, 95% CI 1.82-6.32, p < 0.001) increased the risk of MACE. Regarding the secondary prevention group, only GLP-1 RA cessation by time (HR 2.71, 95% CI 1.46-5.01, p = 0.002) increased the risk of MACE. With respect to those who withdrew treatment, subjects who continued the GLP-1 RA had significantly greater weight loss and lower glycated hemoglobin levels during follow-up.
Conclusions: In this real-world type 2 diabetes population, discontinuation of GLP-1 RA treatment was associated to a higher risk of major cardiovascular events, in both subjects with and without a history of CV events.
Keywords: Cardiovascular events; Diabetes; GLP-1 receptor agonists; Real-world.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests regarding this work.
References
-
- Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91(1):301–307. doi: 10.1172/JCI116186. - DOI - PMC - PubMed
-
- Nauck MA, Kleine N, Ørskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia [Internet]. 1993 [cited 2022 Oct 1];36(8):741–4. Available from: https://pubmed.ncbi.nlm.nih.gov/8405741/ - PubMed
-
- Nauck MA, Heimesaat MM, Behle K, Holst JJ, Nauck MS, Ritzel R, et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87(3):1239–1246. doi: 10.1210/jcem.87.3.8355. - DOI - PubMed
-
- Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–57. - PubMed