The Imbalance of p53-Park7 Signaling Axis Induces Iron Homeostasis Dysfunction in Doxorubicin-Challenged Cardiomyocytes
- PMID: 36967569
- PMCID: PMC10214246
- DOI: 10.1002/advs.202206007
The Imbalance of p53-Park7 Signaling Axis Induces Iron Homeostasis Dysfunction in Doxorubicin-Challenged Cardiomyocytes
Abstract
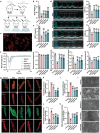
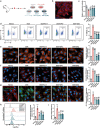
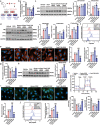
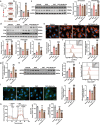
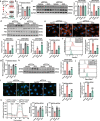
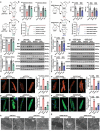
Doxorubicin (DOX)-induced cardiotoxicity (DoIC) is a major side effect for cancer patients. Recently, ferroptosis, triggered by iron overload, is demonstrated to play a role in DoIC. How iron homeostasis is dysregulated in DoIC remains to be elucidated. Here, the authors demonstrate that DOX challenge exhibits reduced contractile function and induction of ferroptosis-related phenotype in cardiomyocytes, evidenced by iron overload, lipid peroxide accumulation, and mitochondrial dysfunction. Compared to Ferric ammonium citrate (FAC) induced secondary iron overload, DOX-challenged cardiomyocytes show a dysfunction of iron homeostasis, with decreased cytoplasmic and mitochondrial iron-sulfur (FeS) cluster-mediated aconitase activity and abnormal expression of iron homeostasis-related proteins. Mechanistically, mass spectrometry analysis identified DOX-treatment induces p53-dependent degradation of Parkinsonism associated deglycase (Park7) which results in iron homeostasis dysregulation. Park7 counteracts iron overload by regulating iron regulatory protein family transcription while blocking mitochondrial iron uptake. Knockout of p53 or overexpression of Park7 in cardiomyocytes remarkably restores the activity of FeS cluster and iron homeostasis, inhibits ferroptosis, and rescues cardiac function in DOX treated animals. These results demonstrate that the iron homeostasis plays a key role in DoIC ferroptosis. Targeting of the newly identified p53-Park7 signaling axis may provide a new approach to prevent DoIC.
Keywords: FeS cluster; doxorubicin-induced cardiotoxicity; ferroptosis; iron homeostasis; p53-Park7 signaling axis.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Miller K. D., Nogueira L., Devasia T., Mariotto A. B., Yabroff K. R., Jemal A., Kramer J., Siegel R. L., Ca‐Cancer J. Clin. 2022, 72, 409. - PubMed
-
- Sawicki K. T., Sala V., Prever L., Hirsch E., Ardehali H., Ghigo A., Annu. Rev. Pharmacol. Toxicol. 2021, 61, 309. - PubMed
-
- Swain S. M., Whaley F. S., Ewer M. S., Cancer 2003, 97, 2869. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
