Response to magnesium sulfate and adrenocorticotropic hormone combination therapy for infantile spasms with failed first-line treatments
- PMID: 36967744
- PMCID: PMC10030695
- DOI: 10.1002/ped4.12368
Response to magnesium sulfate and adrenocorticotropic hormone combination therapy for infantile spasms with failed first-line treatments
Abstract
Importance: Infantile spasm (IS) is a kind of refractory epilepsy. The first-line treatments for IS are adrenocorticotropic hormone (ACTH), oral corticosteroids, and vigabatrin.
Objective: This study aimed to evaluate the efficacy of magnesium sulfate and ACTH (MgSO4+ACTH) combination therapy in patients with IS who failed first-line treatments.
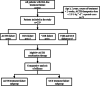
Methods: In this retrospective study, the clinical data of patients with IS who failed first-line treatments were collected in the Chinese PLA General Hospital. Patients received MgSO4+ACTH combination therapy after first-line treatments failed. The course of treatments was 2 weeks. The therapeutic dose of ACTH and MgSO4 was 2.5 U·kg-1·d-1 and 0.25 g·kg-1·d-1, respectively.
Results: A total of 229 patients with IS who failed the first-line treatments were collected. At the end of the MgSO4+ACTH combination treatment, the seizure-free rate was 48.5% (111/229), and the resolution of hypsarrhythmia on electroencephalogram (EEG) was 72.1% (165/229). About 21.4% (49/229) of patients showed side effects, including infectious diseases, hypokalemia, and diarrhea.
Interpretation: For patients with IS who failed first-line treatments, in terms of the seizure-free rate and resolution of hypsarrhythmia on EEG, MgSO4+ACTH combination therapy can be considered.
Keywords: Adrenocorticotropic hormone; Efficacy; Infantile spasms; Magnesium sulfate; Treatment.
© 2023 Chinese Medical Association. Pediatric Investigation published by John Wiley & Sons Australia, Ltd on behalf of Futang Research Center of Pediatric Development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Go CY, Mackay MT, Weiss SK, Stephens D, Adams‐Webber T, Ashwal S, et al. Evidence‐based guideline update: medical treatment of infantile spasms. Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2012;78:1974‐1980. DOI: 10.1212/WNL.0b013e318259e2cf - DOI - PMC - PubMed
-
- Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, et al. The United Kingdom Infantile Spasms Study comparing vigabatrin with prednisolone or tetracosactide at 14 days: a multicentre, randomised controlled trial. Lancet. 2004;364:1773‐1778. DOI: 10.1016/S0140-6736(04)17400-X - DOI - PubMed
-
- O'Callaghan FJ, Edwards SW, Alber FD, Hancock E, Johnson AL, Kennedy CR, et al. Safety and effectiveness of hormonal treatment versus hormonal treatment with vigabatrin for infantile spasms (ICISS): a randomised, multicentre, open‐label trial. Lancet Neurol. 2017;16:33‐42. DOI: 10.1016/S1474-4422(16)30294-0 - DOI - PubMed
LinkOut - more resources
Full Text Sources