Gold nanoparticles enhances radiosensitivity in glioma cells by inhibiting TRAF6/NF-κB induced CCL2 expression
- PMID: 36967939
- PMCID: PMC10036657
- DOI: 10.1016/j.heliyon.2023.e14362
Gold nanoparticles enhances radiosensitivity in glioma cells by inhibiting TRAF6/NF-κB induced CCL2 expression
Abstract
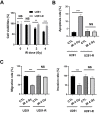
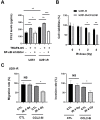
Gliomas are inherently difficult to treat by radiotherapy because glioma cells become radioresistant over time. However, combining radiotherapy with a radiosensitizer could be an effective strategy to mitigate the radioresistance of glioma cells. Gold nanoparticles (AuNPs) have emerged as a promising nanomaterial for cancer therapy, but little is known about whether AuNPs and X-ray radiation have cytotoxic synergistic effects against tumors. In this study, we found that the combination of AuNPs and X-ray irradiation significantly reduced the viabilities, as well as the migration and invasion, of glioma cells. Mechanistically, we observed that the AuNPs inhibited radiation-induced CCL2 expression by inhibiting the TRAF6/NF-κB pathway, which likely manifested the synergistic therapeutic effect between the AuNPs and X-ray radiation. The AuNPs also re-sensitized radioresistant glioma cells by inhibiting CCL2 expression. These results were also observed in another tumor cell line with a different molecular pattern, indicating that the underlying mechanism may be ubiquitous through cancer cells. Lastly, using the glioma mouse model, we observed that AuNPs significantly reduced tumor growth in the presence of X-ray radiation compared to radiotherapy alone.
Keywords: Glioma; Gold nanoparticles; Migration and invasion radio-resistance; Synergistic effect.
© 2023 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Use of DNA-generated gold nanoparticles to radiosensitize and eradicate radioresistant glioma stem cells.Nanotechnology. 2019 Feb 1;30(5):055101. doi: 10.1088/1361-6528/aaedd5. Epub 2018 Nov 30. Nanotechnology. 2019. PMID: 30499457
-
Activation of PTGS2/NF-κB signaling pathway enhances radiation resistance of glioma.Cancer Med. 2019 Mar;8(3):1175-1185. doi: 10.1002/cam4.1971. Epub 2019 Feb 10. Cancer Med. 2019. PMID: 30740906 Free PMC article.
-
Photothermal therapy using folate conjugated gold nanoparticles enhances the effects of 6MV X-ray on mouth epidermal carcinoma cells.J Photochem Photobiol B. 2017 Jul;172:52-60. doi: 10.1016/j.jphotobiol.2017.05.012. Epub 2017 May 12. J Photochem Photobiol B. 2017. PMID: 28527427
-
Radiosensitization by gold nanoparticles: Will they ever make it to the clinic?Radiother Oncol. 2017 Sep;124(3):344-356. doi: 10.1016/j.radonc.2017.07.007. Epub 2017 Aug 4. Radiother Oncol. 2017. PMID: 28784439 Review.
-
Monte Carlo studies in Gold Nanoparticles enhanced radiotherapy: The impact of modelled parameters in dose enhancement.Phys Med. 2020 Dec;80:57-64. doi: 10.1016/j.ejmp.2020.09.022. Epub 2020 Oct 25. Phys Med. 2020. PMID: 33115700 Review.
Cited by
-
Gold Nanoparticle Inhibits the Tumor-Associated Macrophage M2 Polarization by Inhibiting m6A Methylation-Dependent ATG5/Autophagy in Prostate Cancer.Anal Cell Pathol (Amst). 2025 Jan 4;2025:6648632. doi: 10.1155/ancp/6648632. eCollection 2025. Anal Cell Pathol (Amst). 2025. PMID: 39802931 Free PMC article.
-
Atractylenolide II regulates the proliferation, ferroptosis, and immune escape of hepatocellular carcinoma cells by inactivating the TRAF6/NF-κB pathway.Naunyn Schmiedebergs Arch Pharmacol. 2024 Oct;397(10):7697-7710. doi: 10.1007/s00210-024-03046-2. Epub 2024 May 6. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38709266
-
The Recent Research Progress of NF-κB Signaling on the Proliferation, Migration, Invasion, Immune Escape and Drug Resistance of Glioblastoma.Int J Mol Sci. 2023 Jun 19;24(12):10337. doi: 10.3390/ijms241210337. Int J Mol Sci. 2023. PMID: 37373484 Free PMC article. Review.
-
Targeting Cancer Cell Fate: Apoptosis, Autophagy, and Gold Nanoparticles in Treatment Strategies.Curr Issues Mol Biol. 2025 Jun 14;47(6):460. doi: 10.3390/cimb47060460. Curr Issues Mol Biol. 2025. PMID: 40699859 Free PMC article. Review.
-
Nanoradiosensitizers in glioblastoma treatment: recent advances and future perspectives.Nanomedicine (Lond). 2024;19(26):2229-2249. doi: 10.1080/17435889.2024.2395238. Epub 2024 Sep 23. Nanomedicine (Lond). 2024. PMID: 39311492 Review.
References
-
- Liu C., Wang L., Qiu H., Dong Q., Feng Y., Li D., et al. Combined strategy of radioactive 125I seeds and salinomycin for enhanced glioma chemo-radiotherapy: evidences for ROS-mediated apoptosis and signaling crosstalk. Neurochem. Res. 2018;43:1317–1327. doi: 10.1007/s11064-018-2547-2. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

