Research progress of catalysts for aldol condensation of biomass based compounds
- PMID: 36968059
- PMCID: PMC10034479
- DOI: 10.1039/d3ra00906h
Research progress of catalysts for aldol condensation of biomass based compounds
Abstract
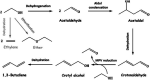
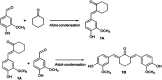
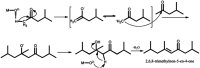
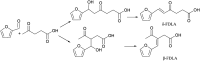
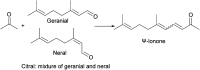
Research progress of catalysts of the aldol condensation reaction of biomass based compounds is summarized for the synthesis of liquid fuel precursors and chemicals. In summary, an acidic catalyst, alkaline catalyst, acid-base amphoteric catalyst, ionic liquid and other catalysts can catalyze the aldol condensation reaction. The aldol condensation reaction catalyzed by an acid catalyst has the problems of low conversion and low yield. The basic catalyst catalyzes the aldol condensation reaction with high conversion and yield, but the existence of liquid alkali is difficult to separate from the product. The reaction temperature needed for oxide and hydrotalcite alkali is relatively high. The basic resin has good catalytic activity and at a low reaction temperature, and is easy to separate from the target product. Acid-base amphoteric catalysts have received extensive attention from researchers for their excellent activity and selectivity. Ionic liquid is a new type of material, which can also be used for the aldol condensation reaction. In the future application of aldol condensation, the development of strong alkaline resin is a good research direction.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
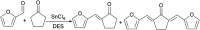

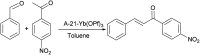









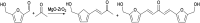





References
-
- Huber G. W. Iborra S. Corma A. Chem. Rev. 2006;106:4044–4098. - PubMed
-
- Corma A. de la Torre O. Renz M. Energy Environ. Sci. 2012;5:6328–6344.
-
- Liu Y. Li G. Hu Y. Wang A. Lu F. Zou J.-J. Cong Y. Li N. Zhang T. Joule. 2019;3:1028–1036.
-
- Bohre A. Alam M. I. Avasthi K. Ruiz-Zepeda F. Likozar B. Appl. Catal., B. 2020;276:119069.
-
- Li S. Li N. Li G. Li L. Wang A. Cong Y. Wang X. Xu G. Zhang T. Appl. Catal., B. 2015;170–171:124–134.
Publication types
LinkOut - more resources
Full Text Sources

