Tissue-specific heteroplasmy segregation is accompanied by a sharp mtDNA decline in Caenorhabditis elegans soma
- PMID: 36968071
- PMCID: PMC10031119
- DOI: 10.1016/j.isci.2023.106349
Tissue-specific heteroplasmy segregation is accompanied by a sharp mtDNA decline in Caenorhabditis elegans soma
Abstract
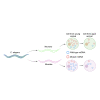
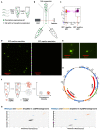
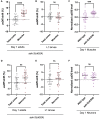
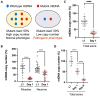
Mutations in the mitochondrial genome (mtDNA) can be pathogenic. Owing to the multi-copy nature of mtDNA, wild-type copies can compensate for the effects of mutant mtDNA. Wild-type copies available for compensation vary depending on the mutant load and the total copy number. Here, we examine both mutant load and copy number in the tissues of Caenorhabditis elegans. We found that neurons, but not muscles, have modestly higher mutant load than rest of the soma. We also uncovered different effect of aak-2 knockout on the mutant load in the two tissues. The most surprising result was a sharp decline in somatic mtDNA content over time. The scale of the copy number decline surpasses the modest shifts in mutant load, suggesting that it may exert a substantial effect on mitochondrial function. In summary, measuring both the copy number and the mutant load provides a more comprehensive view of the mutant mtDNA dynamics.
Keywords: Biological sciences; Cell biology; Molecular biology.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

