Role of exosomal ncRNAs released by M2 macrophages in tumor progression of gastrointestinal cancers
- PMID: 36968082
- PMCID: PMC10031147
- DOI: 10.1016/j.isci.2023.106333
Role of exosomal ncRNAs released by M2 macrophages in tumor progression of gastrointestinal cancers
Abstract
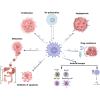
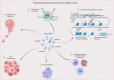
Macrophages (MΦs) type 2 (M2) play crucial roles in the pathogenesis of gastrointestinal cancers (GIC) by enhancing tumor progression, invasion, and metastasis. Polarized M2 has been linked to the increase of GIC tumorigenesis and drug resistance. Several studies reported that M2-derived exosomal non-coding RNAs (Exos-ncRNAs) play pivotal roles in the modulation of the GIC tumor microenvironment (TME) and mostly promote drug resistance and immunosuppression. The impact of M2-Exos-ncRNAs is attributed to altered signaling pathways, enhancement of immunoregulatory mechanisms, and post-transcriptional modulation. Recent studies described novel targets in M2-TAMs-derived Exos-ncRNAs and potential promising clinical outcomes such as inhibiting tumor formation, invasion, and metastasis. Highlighting current knowledge of M2-Exos-ncRNAs involved in GIC pathogenesis and immunomodulation would thus be a significant contribution to improving clinical outcomes. In this review, we summarize recent updates on the role of M2-TAMs-Exos-ncRNAs in GIC pathogenesis, immunosuppression, and drug resistance. A deep understanding of M2-TAMs-derived Exos-ncRNAs could help to identify potential biomarkers and therapeutic targets.
Keywords: Cancer; Immunology.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that there are no financial or nonfinancial conflicts of interest related to this work.
Figures


Similar articles
-
Exosomal ncRNAs facilitate interactive 'dialogue' between tumor cells and tumor-associated macrophages.Cancer Lett. 2023 Jan 1;552:215975. doi: 10.1016/j.canlet.2022.215975. Epub 2022 Oct 25. Cancer Lett. 2023. PMID: 36306940 Review.
-
Therapeutic exosomal vaccine for enhanced cancer immunotherapy by mediating tumor microenvironment.iScience. 2021 Dec 18;25(1):103639. doi: 10.1016/j.isci.2021.103639. eCollection 2022 Jan 21. iScience. 2021. PMID: 35024580 Free PMC article.
-
Noncoding RNAs: Regulating the crosstalk between tumor-associated macrophages and gastrointestinal cancer.Biomed Pharmacother. 2022 Sep;153:113370. doi: 10.1016/j.biopha.2022.113370. Epub 2022 Jul 2. Biomed Pharmacother. 2022. PMID: 35792392 Review.
-
Exosomal miR-27a-3p derived from tumor-associated macrophage suppresses propranolol sensitivity in infantile hemangioma.Cell Immunol. 2021 Dec;370:104442. doi: 10.1016/j.cellimm.2021.104442. Epub 2021 Sep 24. Cell Immunol. 2021. PMID: 34634611
-
Tumor-derived or non-tumor-derived exosomal noncodingRNAs and signaling pathways in tumor microenvironment.Int Immunopharmacol. 2022 May;106:108626. doi: 10.1016/j.intimp.2022.108626. Epub 2022 Feb 18. Int Immunopharmacol. 2022. PMID: 35189470 Review.
Cited by
-
The association of appendectomy with prognosis and tumor-associated macrophages in patients with colorectal cancer.iScience. 2024 Jul 25;27(9):110578. doi: 10.1016/j.isci.2024.110578. eCollection 2024 Sep 20. iScience. 2024. PMID: 39224521 Free PMC article.
References
-
- Kanda M., Mizuno A., Fujii T., Shimoyama Y., Yamada S., Tanaka C., Kobayashi D., Koike M., Iwata N., Niwa Y., et al. Tumor infiltrative pattern predicts sites of recurrence after curative gastrectomy for stages 2 and 3 gastric cancer. Ann. Surg Oncol. 2016;23:1934–1940. doi: 10.1245/s10434-016-5102-x. - DOI - PubMed
-
- Kolbeinsson H., Hoppe A., Bayat A., Kogelschatz B., Mbanugo C., Chung M., Wolf A., Assifi M.M., Wright G.P. Recurrence patterns and postrecurrence survival after curative intent resection for pancreatic ductal adenocarcinoma. Surgery. 2021;169:649–654. doi: 10.1016/j.surg.2020.06.042. - DOI - PubMed
-
- Vakiani E., Shah R.H., Berger M.F., Makohon-Moore A.P., Reiter J.G., Ostrovnaya I., Attiyeh M.A., Cercek A., Shia J., Iacobuzio-Donahue C.A., et al. Local recurrences at the anastomotic area are clonally related to the primary tumor in sporadic colorectal carcinoma. Oncotarget. 2017;8:42487–42494. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

