Single-Molecule Optical Biosensing: Recent Advances and Future Challenges
- PMID: 36968450
- PMCID: PMC10037498
- DOI: 10.1021/acsphyschemau.2c00061
Single-Molecule Optical Biosensing: Recent Advances and Future Challenges
Abstract
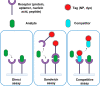
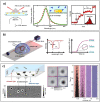
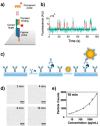
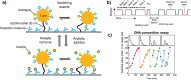
In recent years, the sensitivity and specificity of optical sensors has improved tremendously due to improvements in biochemical functionalization protocols and optical detection systems. As a result, single-molecule sensitivity has been reported in a range of biosensing assay formats. In this Perspective, we summarize optical sensors that achieve single-molecule sensitivity in direct label-free assays, sandwich assays, and competitive assays. We describe the advantages and disadvantages of single-molecule assays and summarize future challenges in the field including their optical miniaturization and integration, multimodal sensing capabilities, accessible time scales, and compatibility with real-life matrices such as biological fluids. We conclude by highlighting the possible application areas of optical single-molecule sensors that include not only healthcare but also the monitoring of the environment and industrial processes.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Parlak O. Portable and wearable real-time stress monitoring: A critical review. Sensors and Actuators Reports 2021, 3, 100036.10.1016/j.snr.2021.100036. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
