Maternal ART throughout gestation prevents caudate volume reductions in neonates who are HIV exposed but uninfected
- PMID: 36968507
- PMCID: PMC10035579
- DOI: 10.3389/fnins.2023.1085589
Maternal ART throughout gestation prevents caudate volume reductions in neonates who are HIV exposed but uninfected
Abstract
Introduction: Successful programmes for prevention of vertical HIV transmission have reduced the risk of infant HIV infection in South Africa from 8% in 2008 to below 1% in 2018/2019, resulting in an increasing population of children exposed to HIV perinatally but who are uninfected (HEU). However, the long-term effects of HIV and antiretroviral treatment (ART) exposure on the developing brain are not well understood. Whereas children who are HEU perform better than their HIV-infected counterparts, they demonstrate greater neurodevelopmental delay than children who are HIV unexposed and uninfected (HUU), especially in resource-poor settings. Here we investigate subcortical volumetric differences related to HIV and ART exposure in neonates.
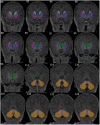
Methods: We included 120 infants (59 girls; 79 HEU) born to healthy women with and without HIV infection in Cape Town, South Africa, where HIV sero-prevalence approaches 30%. Of the 79 HEU infants, 40 were exposed to ART throughout gestation (i.e., mothers initiated ART pre conception; HEU-pre), and 39 were exposed to ART for part of gestation (i.e., mothers initiated ART post conception; HEU-post). Post-conception mothers had a mean (± SD) gestational age (GA) of 15.4 (± 5.7) weeks at ART initiation. Mothers with HIV received standard care fixed drug combination ART (Tenofovir/Efavirenz/Emtricitabine). Infants were imaged unsedated on a 3T Skyra (Siemens, Erlangen, Germany) at mean GA equivalent of 41.5 (± 1.0) weeks. Selected regions (caudate, putamen, pallidum, thalamus, cerebellar hemispheres and vermis, and corpus callosum) were manually traced on T1-weighted images using Freeview.
Results: HEU neonates had smaller left putamen volumes than HUU [β (SE) = -90.3 (45.3), p = 0.05] and caudate volume reductions that depended on ART exposure duration in utero. While the HEU-pre group demonstrated no caudate volume reductions compared to HUU, the HEU-post group had smaller caudate volumes bilaterally [β (SE) = -145.5 (45.1), p = 0.002, and -135.7 (49.7), p = 0.008 for left and right caudate, respectively].
Discussion: These findings from the first postnatal month suggest that maternal ART throughout gestation is protective to the caudate nuclei. In contrast, left putamens were smaller across all HEU newborns, despite maternal ART.
Keywords: HIV exposure; antiretroviral therapy; brain structure; magnetic resonance imaging; neonate; neurodevelopment.
Copyright © 2023 Ibrahim, Warton, Fry, Cotton, Jacobson, Jacobson, Molteno, Little, van der Kouwe, Laughton, Meintjes and Holmes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Alimenti A., Forbes J. C., Oberlander T. F., Money D. M., Grunau R. E., Papsdorf M. P., et al. (2006). A prospective controlled study of neurodevelopment in HIV-uninfected children exposed to combination antiretroviral drugs in pregnancy. Pediatrics 118 e1139–e1145. 10.1542/peds.2006-0525 - DOI - PubMed
LinkOut - more resources
Full Text Sources

