Loss of action-related function and connectivity in the blind extrastriate body area
- PMID: 36968509
- PMCID: PMC10035577
- DOI: 10.3389/fnins.2023.973525
Loss of action-related function and connectivity in the blind extrastriate body area
Abstract
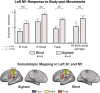
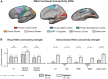
The Extrastriate Body Area (EBA) participates in the visual perception and motor actions of body parts. We recently showed that EBA's perceptual function develops independently of visual experience, responding to stimuli with body-part information in a supramodal fashion. However, it is still unclear if the EBA similarly maintains its action-related function. Here, we used fMRI to study motor-evoked responses and connectivity patterns in the congenitally blind brain. We found that, unlike the case of perception, EBA does not develop an action-related response without visual experience. In addition, we show that congenital blindness alters EBA's connectivity profile in a counterintuitive way-functional connectivity with sensorimotor cortices dramatically decreases, whereas connectivity with perception-related visual occipital cortices remains high. To the best of our knowledge, we show for the first time that action-related functions and connectivity in the visual cortex could be contingent on visuomotor experience. We further discuss the role of the EBA within the context of visuomotor control and predictive coding theory.
Keywords: body representation; congenital blindness; extrastriate body area; neuroimaging; plasticity; resting-state fMRI; visuomotor interactions.
Copyright © 2023 Yizhar, Tal and Amedi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

