Systems prediction of chronic lung allograft dysfunction: Results and perspectives from the Cohort of Lung Transplantation and Systems prediction of Chronic Lung Allograft Dysfunction cohorts
- PMID: 36968829
- PMCID: PMC10033762
- DOI: 10.3389/fmed.2023.1126697
Systems prediction of chronic lung allograft dysfunction: Results and perspectives from the Cohort of Lung Transplantation and Systems prediction of Chronic Lung Allograft Dysfunction cohorts
Abstract
Background: Chronic lung allograft dysfunction (CLAD) is the leading cause of poor long-term survival after lung transplantation (LT). Systems prediction of Chronic Lung Allograft Dysfunction (SysCLAD) aimed to predict CLAD.
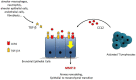
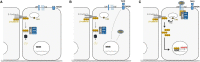
Methods: To predict CLAD, we investigated the clinicome of patients with LT; the exposome through assessment of airway microbiota in bronchoalveolar lavage cells and air pollution studies; the immunome with works on activation of dendritic cells, the role of T cells to promote the secretion of matrix metalloproteinase-9, and subpopulations of T and B cells; genome polymorphisms; blood transcriptome; plasma proteome studies and assessment of MSK1 expression.
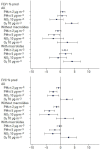
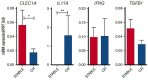
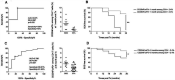
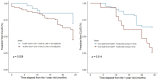
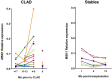
Results: Clinicome: the best multivariate logistic regression analysis model for early-onset CLAD in 422 LT eligible patients generated a ROC curve with an area under the curve of 0.77. Exposome: chronic exposure to air pollutants appears deleterious on lung function levels in LT recipients (LTRs), might be modified by macrolides, and increases mortality. Our findings established a link between the lung microbial ecosystem, human lung function, and clinical stability post-transplant. Immunome: a decreased expression of CLEC1A in human lung transplants is predictive of the development of chronic rejection and associated with a higher level of interleukin 17A; Immune cells support airway remodeling through the production of plasma MMP-9 levels, a potential predictive biomarker of CLAD. Blood CD9-expressing B cells appear to favor the maintenance of long-term stable graft function and are a potential new predictive biomarker of BOS-free survival. An early increase of blood CD4 + CD57 + ILT2+ T cells after LT may be associated with CLAD onset. Genome: Donor Club cell secretory protein G38A polymorphism is associated with a decreased risk of severe primary graft dysfunction after LT. Transcriptome: blood POU class 2 associating factor 1, T-cell leukemia/lymphoma domain, and B cell lymphocytes, were validated as predictive biomarkers of CLAD phenotypes more than 6 months before diagnosis. Proteome: blood A2MG is an independent predictor of CLAD, and MSK1 kinase overexpression is either a marker or a potential therapeutic target in CLAD.
Conclusion: Systems prediction of Chronic Lung Allograft Dysfunction generated multiple fingerprints that enabled the development of predictors of CLAD. These results open the way to the integration of these fingerprints into a predictive handprint.
Keywords: Frontiers in medicine; bronchiolitis obliterans syndrome; chronic lung allograft dysfunction; chronic rejection; pulmonary section; restrictive allograft syndrome.
Copyright © 2023 Pison, Tissot, Bernasconi, Royer, Roux, Koutsokera, Coiffard, Renaud-Picard, Le Pavec, Mordant, Demant, Villeneuve, Mornex, Nemska, Frossard, Brugière, Siroux, Marsland, Foureau, Botturi, Durand, Pellet, Danger, Auffray, Brouard, Nicod, Magnan and Members of the COhort of Lung Transplantation and Systems prediction of Chronic Lung Allograft Dysfunction consortia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









References
-
- Leard LE, Holm AM, Valapour M, Glanville AR, Attawar S, Aversa M, et al. . Consensus document for the selection of lung transplant candidates: an update from the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. (2021) 40:1349–79. doi: 10.1016/j.healun.2021.07.005, PMID: - DOI - PMC - PubMed
-
- Chambers DC, Perch M, Zuckermann A, Cherikh WS, Harhay MO, Hayes D, et al. . The international thoracic organ transplant registry of the International Society for Heart and Lung Transplantation: thirty-eighth adult lung transplantation report - 2021; focus on recipient characteristics. J Heart Lung Transplant. (2021) 40:1060–72. doi: 10.1016/j.healun.2021.07.021, PMID: - DOI - PMC - PubMed
-
- Verleden GM, Glanville AR, Lease ED, Fisher AJ, Calabrese F, Corris PA, et al. . Chronic lung allograft dysfunction: definition, diagnostic criteria, and approaches to treatment-a consensus report from the pulmonary council of the ISHLT. J Heart Transplant. (2019) 38:493–503. doi: 10.1016/j.healun.2019.03.009, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

