Impact of ompk36 genotype and KPC subtype on the in vitro activity of ceftazidime/avibactam, imipenem/relebactam and meropenem/vaborbactam against KPC-producing K. pneumoniae clinical isolates
- PMID: 36968951
- PMCID: PMC10035640
- DOI: 10.1093/jacamr/dlad022
Impact of ompk36 genotype and KPC subtype on the in vitro activity of ceftazidime/avibactam, imipenem/relebactam and meropenem/vaborbactam against KPC-producing K. pneumoniae clinical isolates
Abstract
Objectives: The availability of new β-lactam/β-lactamase inhibitors ceftazidime/avibactam, meropenem/vaborbactam and imipenem/relebactam have redefined contemporary treatment of Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae (KPC-Kp) infections. We aimed to characterize and contrast the in vitro activity of these agents against genetically diverse KPC-Kp clinical isolates.
Methods: We analysed genomes of 104 non-consecutive KPC-Kp isolates and compared the in vitro antibiotic activity by KPC subtype and ompK36 genotype. MICs were determined in triplicate by CLSI methods. Twenty representative isolates were selected for time-kill analyses against physiological steady-state and trough concentrations, as well as 4× MIC for each agent.
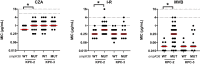
Results: Fifty-eight percent and 42% of isolates harboured KPC-2 and KPC-3, respectively. OmpK36 mutations were more common among KPC-2- compared with KPC-3-producing Kp (P < 0.0001); mutations were classified as IS5 insertion, glycine-aspartic acid insertion at position 134 (GD duplication) and other mutations. Compared to isolates with WT ompK36, ceftazidime/avibactam, imipenem/relebactam and meropenem/vaborbactam MICs were elevated for isolates with IS5 by 2-, 4- and 16-fold, respectively (P < 0.05 for each). Against isolates with GD duplication, imipenem/relebactam and meropenem/vaborbactam MICs were increased, but ceftazidime/avibactam MICs were not. In time-kill studies, ceftazidime/avibactam-mediated killing correlated with ceftazidime/avibactam MICs, and did not vary across ompK36 genotypes. Imipenem/relebactam was not bactericidal against any isolate at trough concentrations. At steady-state imipenem/relebactam concentrations, regrowth occurred more commonly for isolates with IS5 mutations. Log-kills were lower in the presence of meropenem/vaborbactam for isolates with GD duplication compared with IS5 mutations.
Conclusions: Our investigation identified key genotypes that attenuate, to varying degrees, the in vitro activity for each of the new β-lactam/β-lactamase inhibitors. Additional studies are needed to translate the importance of these observations into clinical practice.
© The Author(s) 2023. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures





References
-
- CDC . Antibiotic Resistance Threats in the United States, 2019. 2019.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
