Long-term systemic and mucosal SARS-CoV-2 IgA response and its association with persistent smell and taste disorders
- PMID: 36969158
- PMCID: PMC10031022
- DOI: 10.3389/fimmu.2023.1140714
Long-term systemic and mucosal SARS-CoV-2 IgA response and its association with persistent smell and taste disorders
Abstract
Introduction: Current approved COVID-19 vaccines, notably mRNA and adenoviral vectored technologies, still fail to fully protect against infection and transmission of various SARS-CoV-2 variants. The mucosal immunity at the upper respiratory tract represents the first line of defense against respiratory viruses such as SARS-CoV-2 and is thus critical to develop vaccine blocking human-to-human transmission.
Methods: We measured systemic and mucosal Immunoglobulin A (IgA) response in serum and saliva from 133 healthcare workers from Percy teaching military hospital following a mild infection (SARS-CoV-2 Wuhan strain, n=58) or not infected (n=75), and after SARS-CoV-2 vaccination (Vaxzevria®/Astrazeneca and/or Comirnaty®/Pfizer).
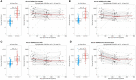
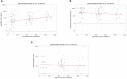
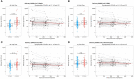
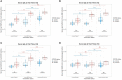
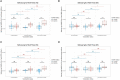
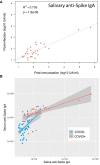
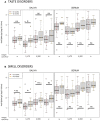
Results: While serum anti-SARS-CoV-2 Spike IgA response lasted up to 16 months post-infection, IgA response in saliva had mostly fallen to baseline level at 6 months post-infection. Vaccination could reactivate the mucosal response generated by prior infection, but failed to induce a significant mucosal IgA response by itself. Early post-COVID-19 serum anti-Spike-NTD IgA titer correlated with seroneutralization titers. Interestingly, its saliva counterpart positively correlated with persistent smell and taste disorders more than one year after mild COVID-19.
Discussion: As breakthrough infections have been correlated with IgA levels, other vaccine platforms inducing a better mucosal immunity are needed to control COVID-19 infection in the future. Our results encourage further studies to explore the prognosis potential of anti-Spike-NTD IgA in saliva at predicting persistent smell and taste disorders.
Keywords: IgA; SARS-CoV2 vaccine; Spike N-terminal domain; dysgeusia; dysosmia; mucosal immunity; smell; taste.
Copyright © 2023 Denis, Garnier, Cheutin, Ferrier, Timera, Jarjaval, Hejl, Billon-Denis, Percy ImmunoCovid group, Ricard, Tournier, Trignol and Mura.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Comparison of SARS-CoV-2 spike-specific IgA and IgG in nasal secretions, saliva and serum.Front Immunol. 2024 Mar 15;15:1346749. doi: 10.3389/fimmu.2024.1346749. eCollection 2024. Front Immunol. 2024. PMID: 38558811 Free PMC article.
-
Systemic and mucosal IgA responses are variably induced in response to SARS-CoV-2 mRNA vaccination and are associated with protection against subsequent infection.Mucosal Immunol. 2022 May;15(5):799-808. doi: 10.1038/s41385-022-00511-0. Epub 2022 Apr 25. Mucosal Immunol. 2022. PMID: 35468942 Free PMC article.
-
Development of an Oral IgA Response against SARS-CoV-2 Following Immunization with Different COVID-19 Vaccines.Viruses. 2023 Nov 25;15(12):2319. doi: 10.3390/v15122319. Viruses. 2023. PMID: 38140560 Free PMC article.
-
Saliva is suitable for SARS-CoV-2 antibodies detection after vaccination: A rapid systematic review.Front Immunol. 2022 Sep 20;13:1006040. doi: 10.3389/fimmu.2022.1006040. eCollection 2022. Front Immunol. 2022. PMID: 36203571 Free PMC article.
-
Guardians of the oral and nasopharyngeal galaxy: IgA and protection against SARS-CoV-2 infection.Immunol Rev. 2022 Aug;309(1):75-85. doi: 10.1111/imr.13118. Epub 2022 Jul 11. Immunol Rev. 2022. PMID: 35815463 Free PMC article. Review.
Cited by
-
SARS-CoV-2 Humoral and Cellular Immune Responses in People Living with HIV.Vaccines (Basel). 2024 Jun 16;12(6):663. doi: 10.3390/vaccines12060663. Vaccines (Basel). 2024. PMID: 38932392 Free PMC article.
-
Evaluation of anti-vector immune responses to adenovirus-mediated lung gene therapy and modulation by αCD20.Mol Ther Methods Clin Dev. 2024 Jun 24;32(3):101286. doi: 10.1016/j.omtm.2024.101286. eCollection 2024 Sep 12. Mol Ther Methods Clin Dev. 2024. PMID: 39070292 Free PMC article.
-
Lessons for medical countermeasure development from unforeseen outbreaks.Emerg Microbes Infect. 2025 Dec;14(1):2471035. doi: 10.1080/22221751.2025.2471035. Epub 2025 Mar 10. Emerg Microbes Infect. 2025. PMID: 39976365 Free PMC article. Review.
-
A novel and safe SmartCap® SC101 to develop the COVID-19 mRNA vaccine STP2104 inducing potent immune responses in humans.Front Immunol. 2025 Jun 16;16:1571092. doi: 10.3389/fimmu.2025.1571092. eCollection 2025. Front Immunol. 2025. PMID: 40589754 Free PMC article. Clinical Trial.
-
Previous immunity shapes immune responses to SARS-CoV-2 booster vaccination and Omicron breakthrough infection risk.Nat Commun. 2023 Sep 12;14(1):5624. doi: 10.1038/s41467-023-41342-2. Nat Commun. 2023. PMID: 37699890 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

