Case Report: Selexipag in pediatric pulmonary hypertension: Initiation, transition, and titration
- PMID: 36969286
- PMCID: PMC10030494
- DOI: 10.3389/fped.2023.1050508
Case Report: Selexipag in pediatric pulmonary hypertension: Initiation, transition, and titration
Erratum in
-
Corrigendum: Case report: Selexipag in pediatric pulmonary hypertension: initiation, transition, and titration.Front Pediatr. 2023 Aug 21;11:1275389. doi: 10.3389/fped.2023.1275389. eCollection 2023. Front Pediatr. 2023. PMID: 37675395 Free PMC article.
Abstract
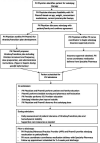
Selexipag, a selective prostacyclin receptor agonist, is approved for treating pulmonary arterial hypertension in WHO Group 1 adult patients. Compared to parenteral prostacyclin formulations, selexipag offers a significant improvement in patient's and caregiver's quality of life because of its oral formulation, frequency of administration, and mechanism of action. Although experience in the pediatric population is limited to case reports in older adolescent patients and selexipag is not approved for use in the pediatric pulmonary hypertension population, many pediatric centers are expanding the use of this therapy to this population. We report our institution's experience in the use of selexipag to treat pulmonary hypertension in children under 10 years of age, between 10 and 30 kg. Seven patients were initiated on selexipag therapy including de novo initiation and transition from intravenous treprostinil to oral selexipag. All patients were on stable background therapy with phosphodiesterase-5 inhibitor and endothelin receptor antagonist therapies at baseline. All patients reached their planned goal selexipag dose during admission without the need for changes to the titration schedule and without hemodynamic deterioration. In our experience, oral selexipag is safe and well-tolerated in young pediatric patients with pulmonary hypertension. Based on our favorable experience, we developed an institution-specific selexipag process algorithm for continued successful use in the pediatric population.
Keywords: initiation; pediatric pulmonary hypertension; prostacyclin; selexipag; transition; treprostinil.
© 2023 Faircloth, Bhatt, Chartan, Coleman, Villafranco, Ruiz, Morales-Demori, Whalen, Ely, Fombin and Varghese.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources