Protein cages as building blocks for superstructures
- PMID: 36969478
- PMCID: PMC9996708
- DOI: 10.1049/enb2.12010
Protein cages as building blocks for superstructures
Abstract
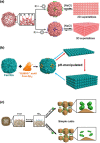
Proteins naturally self-assemble to function. Protein cages result from the self-assembly of multiple protein subunits that interact to form hollow symmetrical structures with functions that range from cargo storage to catalysis. Driven by self-assembly, building elegant higher-order superstructures with protein cages as building blocks has been an increasingly attractive field in recent years. It presents an engineering challenge not only at the molecular level but also at the supramolecular level. The higher-order constructs are proposed to provide access to diverse functional materials. Focussing on design strategy as a perspective, current work on protein cage supramolecular self-assembly are reviewed from three principles that are electrostatic, metal-ligand coordination and inherent symmetry. The review also summarises possible applications of the superstructure architecture built using modified protein cages.
Keywords: molecular biophysics; proteins; self‐assembly.
© 2021 The Authors. Engineering Biology published by John Wiley & Sons Ltd on behalf of The Institution of Engineering and Technology.
Conflict of interest statement
The authors have declared no conflicts of interest for this article.
Figures



References
-
- Uchida, M. , et al.: Biological containers: protein cages as multifunctional nanoplatforms. Adv. Mater. 19(8), 1025–1042 (2007)
-
- Whitesides, G.M. , Grzybowski, B. : Self‐assembly at all scales. Science. 295(5564), 2418–2421 (2002) - PubMed
LinkOut - more resources
Full Text Sources

