An update on SARS-CoV-2 immunization and future directions
- PMID: 36969857
- PMCID: PMC10033701
- DOI: 10.3389/fphar.2023.1125305
An update on SARS-CoV-2 immunization and future directions
Abstract
Millions of people have died as a result of SARS-CoV-2, which was first discovered in China and has since spread globally. Patients with SARS-CoV-2 infection may show a range of symptoms, including fever, coughing, and shortness of breath, or they may show no symptoms at all. To treat COVID-19 symptoms and avoid serious infections, many medications and vaccinations have been employed. However, to entirely eradicate COVID-19 from the world, next-generation vaccine research is required because of the devastating consequences it is having for humanity and every nation's economy. Scientists are working hard to eradicate this dangerous virus across the world. SARS-CoV-2 has also undergone significant mutation, leading to distinct viral types such as the alpha, beta, gamma, delta, and omicron variants. This has sparked discussion about the effectiveness of current vaccines for the newly formed variants. A proper comparison of these vaccinations is required to compare their efficacy as the number of people immunized against SARS-CoV-2 globally increases. Population-level statistics evaluating the capacity of these vaccines to reduce infection are therefore being developed. In this paper, we analyze the many vaccines on the market in terms of their production process, price, dosage needed, and efficacy. This article also discusses the challenges of achieving herd immunity, the likelihood of reinfection, and the importance of convalescent plasma therapy in reducing infection.
Keywords: COVID-19; Moderna; Pfizer; SARS-CoV-2; convalescent plasma therapy; herd immunity; reinfection; vaccine efficacy.
Copyright © 2023 Rana, Kant, Kumra, Gupta, Rana and Ganguly.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

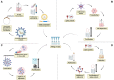
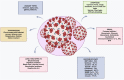
References
-
- Agarwal A., Mukherjee A., Kumar G., Chatterjee P., Bhatnagar T., Malhotra P., et al. (2020). Convalescent plasma in the management of moderate Covid-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID trial). Bmj 371, m3939. 10.1136/bmj.m3939 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

