Role of Hydrogen Sulfide and Hypoxia in Hepatic Angiogenesis of Portal Hypertension
- PMID: 36969894
- PMCID: PMC10037502
- DOI: 10.14218/JCTH.2022.00217
Role of Hydrogen Sulfide and Hypoxia in Hepatic Angiogenesis of Portal Hypertension
Abstract
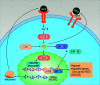
The pathogenesis of portal hypertension remains unclear, and is believed to involve dysfunction of liver sinusoidal endothelial cells (LSEC), activation of hepatic stellate cells (HSC), dysregulation of endogenous hydrogen sulfide (H2S) synthesis, and hypoxia-induced angiogenic responses. H2S, a novel gas transmitter, plays an important role in various pathophysiological processes, especially in hepatic angiogenesis. Inhibition of endogenous H2S synthase by pharmaceutical agents or gene silencing may enhance the angiogenic response of endothelial cells. Hypoxia-inducible factor-1 (HIF-1) is the main transcription factor of hypoxia, which induces hepatic angiogenesis through up-regulation of vascular endothelial growth factor (VEGF) in HSC and LSEC. H2S has also been shown to be involved in the regulation of VEGF-mediated angiogenesis. Therefore, H2S and HIF-1 may be potential therapeutic targets for portal hypertension. The effects of H2S donors or prodrugs on the hemodynamics of portal hypertension and the mechanism of H2S-induced angiogenesis are promising areas for future research.
Keywords: Angiogenesis; Hydrogen sulfide; Hypoxia; Hypoxia-inducible factor; Portal hypertension.
© 2023 Authors.
Conflict of interest statement
The authors have no conflict of interests related to this publication.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
