Conversion of acetate and glyoxylate to fumarate by a cell-free synthetic enzymatic biosystem
- PMID: 36970069
- PMCID: PMC10033897
- DOI: 10.1016/j.synbio.2023.03.004
Conversion of acetate and glyoxylate to fumarate by a cell-free synthetic enzymatic biosystem
Abstract
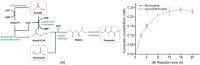
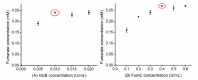
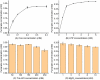
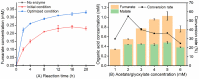
Fumarate is a value-added chemical that is widely used in food, medicine, material, and agriculture industries. With the rising attention to the demand for fumarate and sustainable development, many novel alternative ways that can replace the traditional petrochemical routes emerged. The in vitro cell-free multi-enzyme catalysis is an effective method to produce high value chemicals. In this study, a multi-enzyme catalytic pathway comprising three enzymes for fumarate production from low-cost substrates acetate and glyoxylate was designed. The acetyl-CoA synthase, malate synthase, and fumarase from Escherichia coli were selected and the coenzyme A achieved recyclable. The enzymatic properties and optimization of reaction system were investigated, reaching a fumarate yield of 0.34 mM with a conversion rate of 34% after 20 h of reaction. We proposed and realized the conversion of acetate and glyoxylate to fumarate in vitro using a cell-free multi-enzyme catalytic system, thus providing an alternative approach for the production of fumarate.
Keywords: Acetate; Cell-free; Fumarate; Glyoxylate; Multi-enzyme catalysis.
© 2023 The Authors.
Figures

References
-
- Guo F., Wu M., Dai Z., Zhang S., Zhang W., Dong W., Zhou J., Jiang M., Xin F. Current advances on biological production of fumaric acid. Biochem Eng J. 2020;153
-
- Papadaki A., Androutsopoulos N., Patsalou M., Koutinas M., Kopsahelis N., Castro AMd, Papanikolaou S., Koutinas A.A. Biotechnological production of fumaric acid: the effect of morphology of Rhizopus arrhizus NRRL 2582. Fermentation. 2017;3:33.
-
- Gao C., Guo L., Song W., Wu J., Chen X., Liu L. Advances in microbial engineering for the production of value-added products in a biorefinery. Syst Microbiol Biomanuf. 2022:1–16.
LinkOut - more resources
Full Text Sources

