Tebentafusp: a first-in-class treatment for metastatic uveal melanoma
- PMID: 36970111
- PMCID: PMC10031621
- DOI: 10.1177/17588359231160140
Tebentafusp: a first-in-class treatment for metastatic uveal melanoma
Abstract
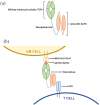
Tebentafusp is a first-in-class immunotherapy agent that comprises an engineered T-cell receptor targeting a gp100 epitope presented by human leukocyte antigen-A*02:01 cells, fused to an anti-CD3 single-chain variable fragment. Tebentafusp is both the first bispecific T-cell engager to show efficacy in the treatment of advanced solid cancer and the first anti-cancer treatment to demonstrate an overall survival benefit in patients with uveal melanoma (UM). This review article will focus on the clinical development of tebentafusp, the mechanism of action and resultant evolution of the management of advanced UM.
Keywords: ImmTAC; gp100; metastatic; tebentafusp; uveal melanoma.
© The Author(s), 2023.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures



References
-
- Kivelä T. The epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and death. Br J Ophthalmol 2009; 93: 1129–1131. - PubMed
-
- Lorigan J, Wallace S, Mavligit G. The prevalence and location of metastases from ocular melanoma: imaging study in 110 patients. AJR Am J Roentgenol 1991; 157: 1279–1281. - PubMed
-
- Kath R, Hayungs J, Bornfeld N, et al.. Prognosis and treatment of disseminated uveal melanoma. Cancer 1993; 72: 2219–2223. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

