Quantification of primary mitral regurgitation by echocardiography: A practical appraisal
- PMID: 36970355
- PMCID: PMC10036770
- DOI: 10.3389/fcvm.2023.1107724
Quantification of primary mitral regurgitation by echocardiography: A practical appraisal
Abstract
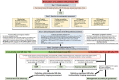
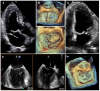
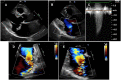
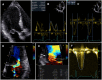
The accurate quantification of primary mitral regurgitation (MR) and its consequences on cardiac remodeling is of paramount importance to determine the best timing for surgery in these patients. The recommended echocardiographic grading of primary MR severity relies on an integrated multiparametric approach. It is expected that the large number of echocardiographic parameters collected would offer the possibility to check the measured values regarding their congruence in order to conclude reliably on MR severity. However, the use of multiple parameters to grade MR can result in potential discrepancies between one or more of them. Importantly, many factors beyond MR severity impact the values obtained for these parameters including technical settings, anatomic and hemodynamic considerations, patient's characteristics and echocardiographer' skills. Hence, clinicians involved in valvular diseases should be well aware of the respective strengths and pitfalls of each of MR grading methods by echocardiography. Recent literature highlighted the need for a reappraisal of the severity of primary MR from a hemodynamic perspective. The estimation of MR regurgitation fraction by indirect quantitative methods, whenever possible, should be central when grading the severity of these patients. The assessment of the MR effective regurgitant orifice area by the proximal flow convergence method should be used in a semi-quantitative manner. Furthermore, it is crucial to acknowledge specific clinical situations in MR at risk of misevaluation when grading severity such as late-systolic MR, bi-leaflet prolapse with multiple jets or extensive leak, wall-constrained eccentric jet or in older patients with complex MR mechanism. Finally, it is debatable whether the 4-grades classification of MR severity would be still relevant nowadays, since the indication for mitral valve (MV) surgery is discussed in clinical practice for patients with 3+ and 4+ primary MR based on symptoms, specific markers of adverse outcome and MV repair probability. Primary MR grading should be seen as a continuum integrating both quantification of MR and its consequences, even for patients with presumed "moderate" MR.
Keywords: echocardiography; heart valve disease (HVD); primary mitral regurgitation; regurgitant fraction; regurgitant volume; valvular regurgitation.
© 2023 Altes, Vermes, Levy, Vancraeynest, Pasquet, Vincentelli, Gerber, Tribouilloy and Marechaux.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

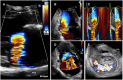
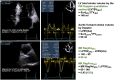

References
Publication types
LinkOut - more resources
Full Text Sources

