A targeted literature review of health economic analyses of human papillomavirus vaccination from various countries
- PMID: 36971102
- PMCID: PMC10273851
- DOI: 10.1177/09564624231165547
A targeted literature review of health economic analyses of human papillomavirus vaccination from various countries
Abstract
Background: Understanding the cost-effectiveness of the HPV vaccine from a global perspective is important to assess from a policy perspective and to support current and future HPV vaccination programs.
Objectives: The aim of this analysis was to conduct a targeted literature review of published pharmacoeconomic literature on the cost-effectiveness of the HPV vaccine to treat patients in various countries, with a focus on cost-savings and their impact on vaccine recommendations.
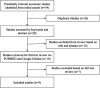
Methods: We searched cost-effectiveness studies in HPV published in peer-reviewed literature from 2012 to 2020 using MEDLINE via the PubMed database and Google Scholar.
Results: HPV vaccine cost-effectiveness was found to be greatest in low-income countries where screen programs were not yet in place, additionally, in adolescent males and females. The majority of the economic evaluations viewed the implementation of the HPV vaccine as cost-effective and recommended national HPV vaccination.
Conclusion: The majority of economic studies favored national HPV vaccination for adolescent males and females in various countries. Feasibility of this strategy and implementation remains an open question, in addition to screening coverage rate in countries with no vaccine programs or countries yet to introduce national HPV vaccination.
Keywords: Human papillomavirus vaccine; cervical cancer prevention; economic analysis; literature review.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
Health economic analysis of human papillomavirus vaccines in women of Chile: perspective of the health care payer using a Markov model.BMC Public Health. 2014 Nov 26;14:1222. doi: 10.1186/1471-2458-14-1222. BMC Public Health. 2014. PMID: 25424716 Free PMC article.
-
Human papillomavirus vaccine introduction in low-income and middle-income countries: guidance on the use of cost-effectiveness models.BMC Med. 2011 May 12;9:54. doi: 10.1186/1741-7015-9-54. BMC Med. 2011. PMID: 21569406 Free PMC article.
-
Cost-effectiveness analysis of the introduction of the human papillomavirus vaccine in Honduras.Vaccine. 2015 May 7;33 Suppl 1:A167-73. doi: 10.1016/j.vaccine.2014.12.067. Vaccine. 2015. PMID: 25919157
-
Should human papillomavirus vaccination target women over age 26, heterosexual men and men who have sex with men? A targeted literature review of cost-effectiveness.Hum Vaccin Immunother. 2018;14(12):3010-3018. doi: 10.1080/21645515.2018.1496878. Epub 2018 Sep 11. Hum Vaccin Immunother. 2018. PMID: 30024823 Free PMC article. Review.
-
The cost-effectiveness of HPV vaccination in addition to screening: a Dutch perspective.Expert Rev Vaccines. 2015 Apr;14(4):589-604. doi: 10.1586/14760584.2014.990386. Epub 2014 Dec 6. Expert Rev Vaccines. 2015. PMID: 25482311 Review.
References
-
- Genital HPV infection – CDC fact sheet . Centers for disease Control and prevention. Washington DC: Department of Health & Human Services, www.cdc.gov/std/hpv/hpv-Fs-July-2017.pdf (2017, accessed 14 January 2022).
-
- HPV infection . Mayo Clinic. Rochester MN: Mayo Foundation for Medical Education and Research (MFMER), www.mayoclinic.org/diseases-conditions/hpv-infection/symptoms-causes/syc... (2021, accessed 27 January 2022).
-
- Smith SS, Hilas O. Human Papillomavirus primer for pharmacists. New York NY: US Pharmacist, www.uspharmacist.com/article/human-papillomavirus-primer-for-pharmacists (2022, accessed 16 December 2022).
-
- Luria L, Cardoza-Favarato G. Human Papillomavirus. StatPearls. Bethesda MD: National Library of Medicine, www.ncbi.nlm.nih.gov/books/NBK448132/ (2022, accessed 12 May 2022).
-
- Hathaway JK. HPV Diagnosis, Prevention, and Treatment. Clin Obstet Gynecol 2012; 55: 671–680. - PubMed