Inhibitory effects of sulfenimides on human and bovine carbonic anhydrase enzymes
- PMID: 36971264
- PMCID: PMC10044159
- DOI: 10.1080/14756366.2023.2194573
Inhibitory effects of sulfenimides on human and bovine carbonic anhydrase enzymes
Abstract
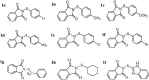
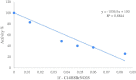
A series of sulfenimide derivatives (1a-i) were investigated as inhibitors of human (hCA-I, hCA-II) and bovine (bCA) carbonic anhydrase enzymes. The compounds were synthesised by the reaction of substituted thiophenols with phthalimide by means of an effective, simple and eco-friendly method and the structures were confirmed by IR, 1H NMR, 13C NMR, MS and elemental analysis. All derivatives except for the methyl derivative (1b) exhibited effective inhibitory action at low micromolar concentrations on human isoforms, but only four derivatives (1e, 1f, 1h, 1i) inhibited the bovine enzyme. The bromo derivative (1f) was found to be strongest inhibitor of all three enzymes with KI values of 0.023, 0.044 and 20.57 µM for hCA-I, hCA-II and bCA, respectively. Results of our study will make valuable contributions to carbonic anhydrase inhibition studies for further investigations since inhibitors of this enzyme are important molecules for medicinal chemistry.
Keywords: Carbonic anhydrase; inhibitor; phthalimide; sulfenimide; thiophenol.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- Supuran CT. Carbonic anhydrase inhibitors. Bioorg Med Chem Lett. 2010;20(12):3467–3474. - PubMed
-
- Del Prete S, Vullo D, Fisher GM, Andrews KT, Poulsen S-A, Capasso C, Supuran CT.. Discovery of a new family of carbonic anhydrases in the malaria pathogen plasmodium falciparum-the η-carbonic anhydrases. Bioorg Med Chem Lett. 2014;24(18):4389–4396. - PubMed
-
- Supuran CT. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov. 2008;7(2):168–181. - PubMed
-
- Arslan T, Celik G, Celik H, Şentürk M, Yaylı N, Ekinci D.. Synthesis and biological evaluation of novel bischalcone derivatives as carbonic anhydrase inhibitors. Arch Pharm. 2016;349(9):741–748. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
