The HSPB1-p62/SQSTM1 functional complex regulates the unconventional secretion and transcellular spreading of the HD-associated mutant huntingtin protein
- PMID: 36971475
- PMCID: PMC10321397
- DOI: 10.1093/hmg/ddad047
The HSPB1-p62/SQSTM1 functional complex regulates the unconventional secretion and transcellular spreading of the HD-associated mutant huntingtin protein
Abstract
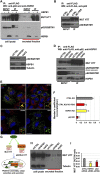
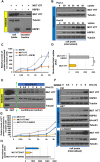
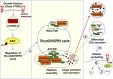
Conformational diseases, such as Alzheimer, Parkinson and Huntington diseases, are part of a common class of neurological disorders characterized by the aggregation and progressive accumulation of proteins bearing aberrant conformations. Huntington disease (HD) has autosomal dominant inheritance and is caused by mutations leading to an abnormal expansion in the polyglutamine (polyQ) tract of the huntingtin (HTT) protein, leading to the formation of HTT inclusion bodies in neurons of affected patients. Interestingly, recent experimental evidence is challenging the conventional view by which the disease pathogenesis is solely a consequence of the intracellular accumulation of mutant protein aggregates. These studies reveal that transcellular transfer of mutated huntingtin protein is able to seed oligomers involving even the wild-type (WT) forms of the protein. To date, there is still no successful strategy to treat HD. Here, we describe a novel functional role for the HSPB1-p62/SQSTM1 complex, which acts as a cargo loading platform, allowing the unconventional secretion of mutant HTT by extracellular vesicles. HSPB1 interacts preferentially with polyQ-expanded HTT compared with the WT protein and affects its aggregation. Furthermore, HSPB1 levels correlate with the rate of mutant HTT secretion, which is controlled by the activity of the PI3K/AKT/mTOR signalling pathway. Finally, we show that these HTT-containing vesicular structures are biologically active and able to be internalized by recipient cells, therefore providing an additional mechanism to explain the prion-like spreading properties of mutant HTT. These findings might also have implications for the turn-over of other disease-associated, aggregation-prone proteins.
© The Author(s) 2023. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures






References
-
- Del Conde, I., Shrimpton, C.N., Thiagarajan, P. and Lopez, J.A. (2005) Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood, 106, 1604–1611. - PubMed
-
- Gatti, S., Bruno, S., Deregibus, M.C., Sordi, A., Cantaluppi, V., Tetta, C. and Camussi, G. (2011) Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol. Dial. Transplant., 26, 1474–1483. - PubMed
-
- Lai, R.C., Arslan, F., Lee, M.M., Sze, N.S., Choo, A., Chen, T.S., Salto-Tellez, M., Timmers, L., Lee, C.N., El Oakley, R.M. et al. (2010) Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res., 4, 214–222. - PubMed
-
- Segura, E., Nicco, C., Bèrangère, L., Vèron, P., Raposo, G., Batteaux, F., Amigorena, S. and Théry, C. (2005) ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood, 106, 216–223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

