A Single Intranasal Dose of Bacterial Therapeutics to Calves Confers Longitudinal Modulation of the Nasopharyngeal Microbiota: a Pilot Study
- PMID: 36971568
- PMCID: PMC10134831
- DOI: 10.1128/msystems.01016-22
A Single Intranasal Dose of Bacterial Therapeutics to Calves Confers Longitudinal Modulation of the Nasopharyngeal Microbiota: a Pilot Study
Abstract
To address the emergence of antimicrobial-resistant pathogens in livestock, microbiome-based strategies are increasingly being sought to reduce antimicrobial use. Here, we describe the effects of intranasal application of bacterial therapeutics (BTs) on the bovine respiratory microbiota and used structural equation modeling to investigate the causal networks after BT application. Beef cattle received (i) an intranasal cocktail of previously characterized BT strains, (ii) an injection of metaphylactic antimicrobial (tulathromycin), or (iii) intranasal saline. Despite being transient colonizers, inoculated BT strains induced longitudinal modulation of the nasopharyngeal bacterial microbiota while showing no adverse effect on animal health. The BT-mediated changes in bacteria included reduced diversity and richness and strengthened cooperative and competitive interactions. In contrast, tulathromycin increased bacterial diversity and antibiotic resistance and disrupted bacterial interactions. Overall, a single intranasal dose of BTs can modulate the bovine respiratory microbiota, highlighting that microbiome-based strategies have potential in being utilized to mitigate bovine respiratory disease in feedlot cattle. IMPORTANCE Bovine respiratory disease (BRD) remains the most significant health challenge affecting the North American beef cattle industry and results in $3 billion in economic losses yearly. Current BRD control strategies mainly rely on antibiotics, with metaphylaxis commonly employed to mitigate BRD incidence in commercial feedlots. However, the emergence of multidrug-resistant BRD pathogens threatens to reduce the efficacy of antimicrobials. Here, we investigated the potential use of novel bacterial therapeutics (BTs) to modulate the nasopharyngeal microbiota in beef calves, which are commonly administered metaphylactic antibiotics to mitigate BRD when sourced from auction markets. By direct comparison of the BTs with an antibiotic commonly used for BRD metaphylaxis in feedlots, this study conveyed the potential use of the BTs to modulate respiratory microbiome and thereby improve resistance against BRD in feedlot cattle.
Keywords: bacterial therapeutics; beef cattle; bovine respiratory pathogens; cattle; intranasal; microbial interactions; respiratory microbiota; structural equation modeling; tulathromycin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
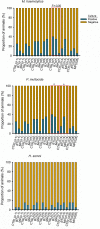
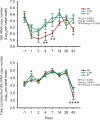

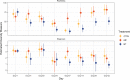
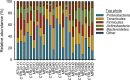
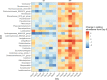
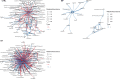
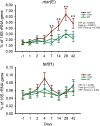
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
