The Photoactive Photosynthetic Reaction Center of a Rhodobacter sphaeroides Mutant Lacking 3-Vinyl (Bacterio)Chlorophyllide a Hydratase Contains 3-Vinyl Bacteriochlorophyll a
- PMID: 36971575
- PMCID: PMC10101016
- DOI: 10.1128/spectrum.03878-22
The Photoactive Photosynthetic Reaction Center of a Rhodobacter sphaeroides Mutant Lacking 3-Vinyl (Bacterio)Chlorophyllide a Hydratase Contains 3-Vinyl Bacteriochlorophyll a
Abstract
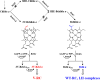
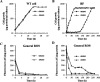
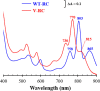
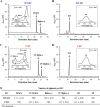
Rhodobacter sphaeroides mutant BF-lacking 3-vinyl (bacterio)chlorophyllide a hydratase (BchF)-accumulates chlorophyllide a (Chlide a) and 3-vinyl bacteriochlorophyllide a (3V-Bchlide a). BF synthesizes 3-vinyl bacteriochlorophyll a (3V-Bchl a) through prenylation of 3V-Bchlide a and assembles a novel reaction center (V-RC) using 3V-Bchl a and Mg-free 3-vinyl bacteriopheophytin a (3V-Bpheo a) at a molar ratio of 2:1. We aimed to verify whether a bchF-deleted R. sphaeroides mutant produces a photochemically active RC that facilitates photoheterotrophic growth. The mutant grew photoheterotrophically-implying a functional V-RC-as confirmed by the emergence of growth-competent suppressors of bchC-deleted mutant (BC) under irradiation. Suppressor mutations in BC were localized to bchF, which diminished BchF activity and caused 3V-Bchlide a accumulation. bchF expression carrying the suppressor mutations in trans resulted in the coproduction of V-RC and wild-type RC (WT-RC) in BF. The V-RC had a time constant (τ) for electron transfer from the primary electron donor P (a dimer of 3V-Bchl a) to the A-side containing 3V-Bpheo a (HA) similar to that of the WT-RC and a 60% higher τ for electron transfer from HA to quinone A (QA). Thus, the electron transfer from HA to QA in the V-RC should be slower than that in the WT-RC. Furthermore, the midpoint redox potential of P/P+ of the V-RC was 33 mV more positive than that of the WT-RC. R. sphaeroides, thus, synthesizes the V-RC when 3V-Bchlide a accumulates. The V-RC can support photoheterotrophic growth; however, its photochemical activity is inferior to that of the WT-RC. IMPORTANCE 3V-Bchlide a is an intermediate in the bacteriochlorophyll a (Bchl a)-specific biosynthetic branch and prenylated by bacteriochlorophyll synthase. R. sphaeroides synthesizes V-RC that absorbs light at short wavelengths. The V-RC was not previously discovered because 3V-Bchlide a does not accumulate during the growth of WT cells synthesizing Bchl a. The levels of reactive oxygen species increased with the onset of photoheterotrophic growth in BF, resulting in a long lag period. Although the inhibitor of BchF is unknown, the V-RC may act as a substitute for the WT-RC when BchF is completely inhibited. Alternatively, it may act synergistically with WT-RC at low levels of BchF activity. The V-RC may broaden the absorption spectra of R. sphaeroides and supplement its photosynthetic ability at various wavelengths of visible light to a greater extent than that by the WT-RC alone.
Keywords: 3-vinyl bacteriochlorophyll a; Rhodobacter sphaeroides; bchF mutation; reaction center.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Struck A, Beese D, Cmiel E, Fischer M, Müller A, Schäfer W, Scheer H. 1990. Modified bacterial reaction centers: 3. Chemically modified chromophores at sites BA, BB and HA, HB, p 313–326. In Michel-Beyerle M-E (ed), Reaction centers of photosynthetic bacteria. Springer, Berlin, Germany.
LinkOut - more resources
Full Text Sources
Miscellaneous

