Inhaled corticosteroids versus placebo for stable chronic obstructive pulmonary disease
- PMID: 36971693
- PMCID: PMC10042218
- DOI: 10.1002/14651858.CD002991.pub4
Inhaled corticosteroids versus placebo for stable chronic obstructive pulmonary disease
Abstract
Background: The role of inhaled corticosteroids (ICS) in chronic obstructive pulmonary disease (COPD) has been the subject of much uncertainty. COPD clinical guidelines currently recommend selective use of ICS. ICS are not recommended as monotherapy for people with COPD, and are only given in combination with long-acting bronchodilators due to greater efficacy of combination therapy. Incorporating and critiquing newly published placebo-controlled trials into the monotherapy evidence base may help to resolve ongoing uncertainties and conflicting findings about their role in this population.
Objectives: To evaluate the benefits and harms of inhaled corticosteroids, used as monotherapy versus placebo, in people with stable COPD, in terms of objective and subjective outcomes.
Search methods: We used standard, extensive Cochrane search methods. The latest search date was October 2022.
Selection criteria: We included randomised trials comparing any dose of any type of ICS, given as monotherapy, with a placebo control in people with stable COPD. We excluded studies of less than 12 weeks' duration and studies of populations with known bronchial hyper-responsiveness (BHR) or bronchodilator reversibility.
Data collection and analysis: We used standard Cochrane methods. Our a priori primary outcomes were 1. exacerbations of COPD and 2. quality of life. Our secondary outcomes were 3. all-cause mortality, 4. lung function (rate of decline of forced expiratory volume in one second (FEV1)), 5. rescue bronchodilator use, 6. exercise capacity, 7. pneumonia and 8. adverse events including pneumonia. ]. We used GRADE to assess certainty of evidence.
Main results: Thirty-six primary studies with 23,139 participants met the inclusion criteria. Mean age ranged from 52 to 67 years, and females were 0% to 46% of participants. Studies recruited across the severities of COPD. Seventeen studies were of duration longer than three months and up to six months and 19 studies were of duration longer than six months. We judged the overall risk of bias as low. Long-term (more than six months) use of ICS as monotherapy reduced the mean rate of exacerbations in those studies where pooling of data was possible (generic inverse variance analysis: rate ratio 0.88 exacerbations per participant per year, 95% confidence interval (CI) 0.82 to 0.94; I2 = 48%, 5 studies, 10,097 participants; moderate-certainty evidence; pooled means analysis: mean difference (MD) -0.05 exacerbations per participant per year, 95% CI -0.07 to -0.02; I2 = 78%, 5 studies, 10,316 participants; moderate-certainty evidence). ICS slowed the rate of decline in quality of life, as measured by the St George's Respiratory Questionnaire (MD -1.22 units/year, 95% CI -1.83 to -0.60; I2 = 0%; 5 studies, 2507 participants; moderate-certainty evidence; minimal clinically importance difference 4 points). There was no evidence of a difference in all-cause mortality in people with COPD (odds ratio (OR) 0.94, 95% CI 0.84 to 1.07; I2 = 0%; 10 studies, 16,636 participants; moderate-certainty evidence). Long-term use of ICS reduced the rate of decline in FEV1 in people with COPD (generic inverse variance analysis: MD 6.31 mL/year benefit, 95% CI 1.76 to 10.85; I2 = 0%; 6 studies, 9829 participants; moderate-certainty evidence; pooled means analysis: 7.28 mL/year, 95% CI 3.21 to 11.35; I2 = 0%; 6 studies, 12,502 participants; moderate-certainty evidence).
Adverse events: in the long-term studies, the rate of pneumonia was increased in the ICS group, compared to placebo, in studies that reported pneumonia as an adverse event (OR 1.38, 95% CI 1.02 to 1.88; I2 = 55%; 9 studies, 14,831 participants; low-certainty evidence). There was an increased risk of oropharyngeal candidiasis (OR 2.66, 95% CI 1.91 to 3.68; 5547 participants) and hoarseness (OR 1.98, 95% CI 1.44 to 2.74; 3523 participants). The long-term studies that measured bone effects generally showed no major effect on fractures or bone mineral density over three years. We downgraded the certainty of evidence to moderate for imprecision and low for imprecision and inconsistency.
Authors' conclusions: This systematic review updates the evidence base for ICS monotherapy with newly published trials to aid the ongoing assessment of their role for people with COPD. Use of ICS alone for COPD likely results in a reduction of exacerbation rates of clinical relevance, probably results in a reduction in the rate of decline of FEV1 of uncertain clinical relevance and likely results in a small improvement in health-related quality of life not meeting the threshold for a minimally clinically important difference. These potential benefits should be weighed up against adverse events (likely to increase local oropharyngeal adverse effects and may increase the risk of pneumonia) and probably no reduction in mortality. Though not recommended as monotherapy, the probable benefits of ICS highlighted in this review support their continued consideration in combination with long-acting bronchodilators. Future research and evidence syntheses should be focused in that area.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
IY: none.
OF: none.
MC: received personal funds as independent contractor for Novo Nordisk.
ES: none.
KF: none.
Figures
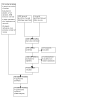
















































Update of
-
Inhaled corticosteroids for stable chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2012 Jul 11;2012(7):CD002991. doi: 10.1002/14651858.CD002991.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2023 Mar 27;3:CD002991. doi: 10.1002/14651858.CD002991.pub4. PMID: 22786484 Free PMC article. Updated.
Comment in
-
Stable COPD and the Role of Inhaled Corticosteroids.Am Fam Physician. 2023 Dec;108(6):Online. Am Fam Physician. 2023. PMID: 38215414 No abstract available.
References
References to studies included in this review
Bourbeau 1998 {published data only}
Bourbeau 2007 {published data only}
Burge 2000 {published data only}
-
- Bale G, Martinez-Camblor P, Burge PS, Soriano JB. Long-term mortality follow-up of the ISOLDE participants: causes of death during 13 years after trial completion. Respiratory Medicine 2008;102(10):1468-72. - PubMed
-
- Calverley PM, Spencer S, Willits L, Burge PS, Jones PW. Withdrawal from treatment as an outcome in the ISOLDE study of COPD. Chest 2003;124(4):1350. - PubMed
Calverley 2003a {published data only}
-
- Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003;361(9356):449-56. - PubMed
-
- Calverley PM, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Safety of salmeterol/fluticasone propionate combination in the treatment of chronic obstructive pulmonary disease. European Respiratory Journal 2002;20(Suppl 38):242s [P1572].
-
- Calverley PM, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Clinical improvements with salmeterol / fluticasone propionate combination in differing severities of COPD [A035] [Poster D50]. www.abstracts2view.com (accessed prior to 11 March 2022).
-
- Calverley PM, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Salmeterol/fluticasone propionate combination for one year provides greater clinical benefit than its individual components. www.abstracts-on-line.com/abstracts/ATS (accessed prior to 11 March 2022).
-
- GSK-SFCB3024. A multicentre, randomised, double-blind, parallel group, placebo-controlled study to compare the efficacy and safety of the salmeterol/FP combination product at a strength of 50/500mcg bd with salmeterol 50mcg bd alone and FP 500mcg bd alone, delivered via the DISKUS™/ACCUHALER™, in the treatment of subjects with chronic obstructive pulmonary disease (COPD) for 12 months. www.gsk-studyregister.com/en/trial-details/?id=SFCB3024 (first received 1 August 1998).
Calverley 2003b {published data only}
-
- Borgstrom L, Asking L, Olsson H, Peterson S. Lack of interaction between disease severity and therapeutic response with budesonide/formoterol in a single inhaler [Abstract]. In: American Thoracic Society 100th international conference; 2004 May 21-26; Orlando. 2004.
-
- Calverley PM, Bonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. European Respiratory Journal 2003;22(6):912-9. - PubMed
-
- Calverley PM, Cseke Z, Peterson S. Budesonide/formoterol reduces the use of oral corticosteroids in the treatment of COPD [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P436. - PubMed
-
- Calverley PM, Kuna P, Olsson H. COPD exacerbations are reduced by budesonide/formoterol in a single inhaler [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P1587.
-
- Calverley PM, Olsson H, Symbicort International COPD Study Group. Budesonide/formoterol in a single inhaler sustains improvements in lung function over 12 months compared with monocomponents and placebo in patients with COPD [abstract]. In: American Thoracic Society 99th International Conference; 2003 May 16-21; Seattle. 2003:B024 Poster 418.
Calverley 2007 {unpublished data only}
-
- Calverley P, Celli B, Anderson JA, Ferguson GT, Jenkins C, Jones PW, et al. The TOwards a Revolution in COPD Health (TORCH) study: salmeterol/fluticasone propionate improves survival in COPD over three years [Abstract]. Respirology 2006;11(Suppl 5):A149 [PS-3-8].
-
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Cardiovascular events in patients with COPD: TORCH study results. Thorax 2010;65(8):719-25. - PubMed
-
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. New England Journal of Medicine 2007;356(8):775-89. - PubMed
-
- Calverley PM, Celli B, Andersen JA, Ferguson GT, Jenkins C, Jones PW, et al. The TORCH (towards a revolution in COPD health) study salmeterol/fluticasone propionate (SFC) improves survival in COPD over three years [Abstract]. European Respiratory Journal 2006;28(Suppl 50):34s [E311].
-
- Calverley PM, Celli B, Anderson JA, Ferguson GT, Jenkins C, Jones PW, Vestbo J, et al. The towards a revolution in COPD health (TORCH) study: fluticasone propionate/salmeterol improves survival in COPD over three years [Abstract]. Chest 2006;130(4):122s.
Calverley 2008 {published data only}
-
- Calverley P, Pauwels R, Nieminem M, Stryszak P, Staudinger H, Lee T. Once-daily mometasone furoate dry powder inhaler preserves lung function, reduces symptoms, and delays exacerbations in patients with COPD previously maintained on ICS. In: European Respiratory Society 13th Annual Congress; 2003 Sep 28-29; Vienna. 2003:P155.
-
- Nelson H, Karpel JP, Staudinger H, Busse WW. Mometasone furoate dry powder inhaler (MF-DPI) improves FEV1, symptoms, quality of life and reduces exacerbations in patients with COPD. Chest 2004;236(4 Suppl):709s-10s.
Derenne 1995 {published data only}
-
- Derenne JP. Effects of high dose inhaled beclomethasone in the rate of decline in FEV1 in patients with chronic obstructive pulmonary disease: results of a 2 years prospective multicentre study. American Journal of Respiratory and Critical Care Medicine 1995;151:A463.
GSK FCO30002 {unpublished data only}
-
- FCO30002. A multicentre, randomised, placebo-controlled, double-blind comparison with 3 parallel groups to investigate the efficacy and safety of inhaled glucocorticoid fluticasone (500 μg bd via Diskus™) vs. oral glucocorticoid therapy vs. placebo in subjects with chronic obstructive airway disease (COPD) under therapy with salmeterol (50 μg bd). GlaxoSmithKline Clinical Trial Register 2005.
GSK FLTA3025 {unpublished data only}
-
- FLTA3025. A randomized, double-blind, parallel-group, comparative trial of inhaled fluticasone propionate 250mcg BID, 500mcg BID, and placebo BID via the DISKUS in subjects with chronic obstructive pulmonary disease (COPD). www.gsk-studyregister.com/en/trial-details/?id=FLTA3025 (first received 8 August 1998).
GSK‐SCO30002 {unpublished data only}
-
- GSK-SCO30002. A multicentre, randomised, double-blind, parallel group, placebo-controlled study to compare the efficacy and safety of inhaled salmeterol/fluticasone propionate combination product 25/250 µg two puffs bd and fluticasone propionate 250µg two puffs bd alone, all administered via metered dose inhalers (MDI), in the treatment of subjects with chronic obstructive pulmonary disease (COPD) for 52 weeks. www.gsk-studyregister.com/en/trial-details/?id=SCO30002 (first received 11 January 2006).
GSK SFCA3007 {published data only}
-
- GSK SFCA3007. A randomized, double-blind, placebo-controlled, parallel-group trial evaluating the safety and efficacy of the DISKUS formulations of salmeterol (SAL) 50mcg BID and fluticasone propionate (FP) 250mcg BID individually and in combination as salmeterol 50mcg/fluticasone propionate 250mcg BID (SFC 50/250) compared to placebo in COPD subjects. www.gsk-studyregister.com/en/trial-details/?id=SFCA3007 (first received 10 November 1998).
-
- Hanania NA, Darken P, Horstman D, Reisner C, Lee B, Davis S, et al. The efficacy and safety of fluticasone propionate (250 micro g)/salmeterol (50 micro g) combined in the Diskus inhaler for the treatment of COPD. Chest 2003;124(3):834-43. - PubMed
-
- Hanania NA, Ramsdell J, Payne K, Davis S, Horstman D, Lee B, et al. Improvements in airflow and dyspnea in COPD patients following 24 weeks treatment with salmeterol 50mcg and fluticasone propionate 250mcg alone or in combination via the Diskus. American Journal of Respiratory and Critical Care Medicine 2001;163(Suppl 5):A279.
-
- Horstman D, Darken P, Davis S, Lee B. Improvements in FEV1 and symptoms in poorly reversible COPD patients following treatment with salmeterol 50mcg/fluticasone propionate 250mcg combination [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P434.
-
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to combination therapy in chronic obstructive pulmonary disease (COPD) [Abstract]. In: US COPD Coalition National COPD Conference 2003; Nov 14-15, Arlington. 2003:1081.
Hattotuwa 2002 {published data only}
-
- Hattotuwa KL, Gizycki MJ, Ansari TW, Jeffery PK, Barnes NC. The effects of inhaled fluticasone on airway inflammation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2002;165(12):1592-9. - PubMed
John 2005 {published data only}
-
- John M, Bosse S, Oltmanns U, Schumacher A, Witt C. Effects of inhaled HFA beclomethasone on pulmonary function and symptoms in patients with chronic obstructive pulmonary disease. Respiratory Medicine 2005;99(11):1418-24. - PubMed
-
- John M, Bosse S, Schumacher A, Oltmanns U, Witt C. Effects of inhaled beclomethasone HFA 134 (qvar/ventoiair) on health related quality of life in patients with chronic obstructive pulmonary disease [Abstract]. In: American Thoracic Society International Conference; 2005 May 20-25; San Diego. 2005:Poster A43.
Kerstjens 1992 {published data only}
-
- Kaptein AA, Brand PL, Dekker FW, Kerstjens HA, Postma DS, Sluiter HJ. Quality-of-life in a long-term multicentre trial in chronic nonspecific lung disease: assessment at baseline. The Dutch CNSLD Study Group. European Respiratory Journal 1993;6(10):1479-84. - PubMed
-
- Kerstjens HA, Brand PL, Hughes MD, Robinson NJ, Postma DS, Sluiter HJ, et al. A comparison of bronchodilator therapy with or without inhaled corticosteroid therapy for obstructive airways disease. New England Journal of Medicine 1992;327(20):1413-9. - PubMed
-
- Kerstjens HA, Overbeek SE, Schouten JP, Brand PL, Postma DS. Airways hyperresponsiveness, bronchodilator response, allergy and smoking predict improvement in FEV1 during long-term inhaled corticosteroid treatment. Dutch CNSLD Study Group. European Respiratory Journal 1993;6(6):868-76. - PubMed
Kerwin 2013 {published data only}
-
- Kerwin EM, Scott-Wilson C, Sanford L, Rennard S, Agusti A, Barnes N, et al. A randomised trial of fluticasone furoate/vilanterol (50/25 mug; 100/25 mug) on lung function in COPD. Respiratory Medicine 2013;107:560-9. - PubMed
Lapperre 2009 {published data only}
-
- Lapperre TS, Snoeck-Stroband JB, Gosman MM, Jansen DF, Schadewijk A, Thiadens HA, et al. Effect of fluticasone with and without salmeterol on pulmonary outcomes in chronic obstructive pulmonary disease: a randomized trial. Annals of Internal Medicine 2009;151(8):517-27. - PubMed
Laptseva 2002 {published data only}
-
- Laptseva IM, Laptseva EA, Borshchevsky VV, Gurevich G, Kalechits O. Inhaled budesonide in the management of chronic obstructive pulmonary disease. European Respiratory Journal 2002;20(Suppl 38):244s.
LHS 2000 {published data only}
-
- Anthonisen NR, Connett JC, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. American Journal of Respiratory and Critical Care Medicine 2002;166(5):675-9. - PubMed
-
- Eichenhorn MS, Wise RA, Madhok TC, Gerald LB, Bailey WC, Tashkin DP, et al. Lack of long-term adverse adrenal effects from inhaled triamcinolone: Lung Health Study II. Chest 2003;124(1):57-62. - PubMed
-
- Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. New England Journal of Medicine 2000;343(26):1902-9. - PubMed
-
- Scanlon PD, Connett JE, Wise RA, Tashkin DP, Madhok T, Skeans M, et al. Loss of one density with inhaled triamcinolone in Lung Health Study II. American Journal of Respiratory and Critical Care Medicine 2004;170(12):1302-9. - PubMed
-
- Tashkin DP, Murray HE, Skeans M, Murray RP. Skin manifestations of inhaled corticosteroids in COPD patients. Chest 2004;126(4):1123-33. - PubMed
Mahler 2002 {published data only}
-
- GSK SFCA3006. A randomized, double-blind, placebo-controlled, parallel-group trial evaluating the safety and efficacy of the DISKUS formulations of salmeterol (SAL) 50mcg BID and fluticasone propionate (FP) 500mcg BID individually and in combination as salmeterol 50mcg/fluticasone propionate 500mcg BID (SFC 50/500) compared to placebo in COPD subjects. www.gsk-studyregister.com/en/trial-details/?id=SFCA3006 (first accessed 24 September 1998).
-
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to combination therapy in chronic obstructive pulmonary disease (COPD). www.abstracts2view.com (accessed prior to 9 September 2022).
-
- Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, et al. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2002;166(8):1084-91. - PubMed
Martinez 2013 {published data only}
-
- Martinez FJ, Boscia J, Feldman G, Scott-Wilson C, Kilbride S, Fabbri L, et al. Fluticasone furoate/vilanterol (100/25; 200/25 mug) improves lung function in COPD: a randomised trial. Respiratory Medicine 2013;107:550-9. - PubMed
Mirici 2001 {published data only}
-
- Mirici A, Bektas Y, Ozbakis G, Erman Z. Effect of inhaled corticosteroids on respiratory function tests and airway inflammation in stable chronic obstructive pulmonary disease. Clinical Drug Investigation 2001;21(12):835-42.
Ozol 2005 {published data only}
-
- Ozol D, Aysan T, Solak ZA, Mogulkoc N, Veral A, Sebik F. The effect of inhaled corticosteroids on bronchoalveolar lavage cells and IL-8 levels in stable COPD patients. Respiratory Medicine 2005;99(12):1494-500. - PubMed
Paggiaro 1998 {published data only}
-
- FLIT97. A multi-centre, randomised, double-blind, parallel group study of the efficacy and safety of inhaled fluticasone propionate 1000µg daily with placebo in chronic obstructive pulmonary disease. ctr.gsk.co.uk 2005.
-
- Paggiaro PL, Dahle R, Bakran I, Frith L, Hollingworth K, Efthimiou J. Multicentre randomised placebo-controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. International COPD Study Group. Lancet 1998;351(9105):773-80. - PubMed
Pauwels 1999 {published data only}
-
- Johnell O, Pauwels R, Löfdahl C-G, Laitinen LA, Postma DS, Pride NB, et al. Bone mineral density in patients with chronic obstructive pulmonary disease treated with budesonide Turbuhaler. European Respiratory Journal 2002;19(6):1058-63. - PubMed
-
- Lofdahl CG, Postma DS, Laitinen LA, Ohlsson SV, Pauwels RA, Pride NB. The European Respiratory Society study on chronic obstructive pulmonary disease (EUROSCOP): recruitment methods and strategies. Respiratory Medicine 1998;92(3):467-72. - PubMed
-
- Pauwels RA, Lofdahl CG, Laitinen LA, Schouten JP, Postma DS, Pride NB, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. New England Journal of Medicine 1999;340(25):1948-53. - PubMed
-
- Pauwels RA, Lofdahl CG, Pride NB, Postma DS, Laitinen LA, Ohlsson SV. European Respiratory Society study on chronic obstructive pulmonary disease (EUROSCOP): hypothesis and design. European Respiratory Journal 1992;5(10):1254-61. - PubMed
Renkema 1996 {published data only}
-
- Renkema TE, Schouten JP, Koeter GH, Postma DS. Effects of long-term treatment with corticosteroids in COPD. Chest 1996;109(5):1156-62. - PubMed
Schermer 2009 {published data only}
-
- Schermer T, Chavannes N, Dekhuijzen R, Wouters E, Muris J, Akkermans R, et al. Fluticasone and N-acetylcysteine in primary care patients with COPD or chronic bronchitis. Respiratory Medicine 2009;103(4):542-51. - PubMed
Senderovitz 1999 {published data only}
-
- Senderovitz T, Vestbo J, Frandsen J, Maltbaek N, Norgaard M, Nielsen C, et al. Steroid reversibility test followed by inhaled budesonide or placebo in outpatients with stable chronic obstructive pulmonary disease. Respiratory Medicine 1999;93(10):715-8. - PubMed
Shaker 2009 {published data only}
-
- Shaker SB, Dirksen A, Ulrik CS, Hestad M, Stavngaard T, Laursen LC, Maltbaek N, et al. The effect of inhaled corticosteroids on the development of emphysema in smokers assessed by annual computed tomography. COPD 2009;6(2):104-11. - PubMed
-
- Shaker SB, Stavngaard T, Laursen LC, Stoel BC, Dirksen A. Rapid fall in lung density following smoking cessation in COPD. COPD 2011;8(1):2-7. - PubMed
Szafranski 2003 {published data only}
-
- Anderson P. Budesonide/formoterol in a single inhaler (Symbicort) provides early and sustained improvement in lung function in moderate to severe COPD. Thorax 2002;57(Suppl III):iii43.
-
- Calverley PM, Thompson NC, Olsson H. Budesonide/formoterol in a single inhaler sustains lung function improvements in COPD [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P435.
-
- Calverley PM. Effect of budesonide/formoterol on severe exacerbations and lung function in moderate to severe COPD. Thorax 2002;57 (Suppl 3):S145.
-
- Campbell LM, Szafranski W. Budesonide/formoterol in a single inhaler (Symbicort) provides sustained relief from symptoms in moderate to severe COPD. In: BTS Winter Meeting, 2002 Dec 4-6; London. 2002:S143.
-
- Campbell LW, Szafranski W. Budesonide/formoterol in a single inhaler (Symbicort) reduces severe exacerbations in patients with moderate-severe COPD. In: BTS Winter Meeting, 2002 Dec 4-6; London. 2002:S141.
Tashkin 2008 {published data only}
-
- Tashkin DP, Rennard SI, Martin P, Ramachandran S, Martin UJ, Silkoff PE, et al. Efficacy and safety of budesonide and formoterol in one pressurized metered-dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease: results of a 6-month randomized clinical trial. Drugs 2008;68(14):1975-2000. - PubMed
Tashkin 2012 {published data only}
-
- Tashkin DP, Doherty DE, Kerwin E, Matiz-Bueno CE, Knorr B, Shekar T, et al. Efficacy and safety characteristics of mometasone furoate/formoterol fumarate fixed-dose combination in subjects with moderate to very severe COPD: findings from pooled analysis of two randomized, 52-week placebo-controlled trials. International Journal of Chronic Obstructive Pulmonary Disease 2012;7:73-86. - PMC - PubMed
Vestbo 1999 {published data only}
-
- Vestbo J, Sorensen T, Lange P, Brix A, Torre P, Viskum K. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 1999;353(9167):1819-23. [MEDLINE: ] - PubMed
Vestbo 2016 {published data only}
-
- Vestbo J, Anderson JA, Brook RD, Calverley PM, Celli BR, Crim C, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet 2016;387:1817-26. - PubMed
Weir 1999 {published data only}
-
- Weir DC, Bale GA, Bright P, Sherwood Burge P. A double-blind placebo-controlled study of the effect of inhaled beclomethasone dipropionate for 2 years in patients with nonasthmatic chronic obstructive pulmonary disease. Clinical and Experimental Allergy Supplement 1999;29(2):125-8. - PubMed
Yildiz 2004 {published data only}
-
- Yildiz F, Basyigit I, Yildirim E, Boyaci H, Ilgazli A. Does addition of inhaled steroids to combined bronchodilator therapy affect health status in patients with COPD? Respirology 2004;9(3):352-5. - PubMed
References to studies excluded from this review
Albers 2004 {published data only}
-
- Albers M, Schermer T, dan Boom G, Akkermans R, Schayck C, Herwaarden C, et al. Efficacy of inhaled steroids in undiagnosed subjects at high risk for COPD: results of the detection, intervention, and monitoring of COPD and asthma program. Chest 2004;126(6):1815-24. - PubMed
Anonymous 1999 {published data only}
-
- Anonymous. Inhaled glucocorticoids do not help smokers with mild chronic obstructive pulmonary disease. Modern Medicine of Australia 1999;42(12):9.
Anonymous 2000 {published data only}
-
- Anonymous. Inhaled corticosteroids: their role in chronic obstructive pulmonary disease. MeReC Bulletin 2000;11(6):21-4.
Auffarth 1991 {published data only}
Balbi 2000 {published data only}
-
- Balbi B, Majori M, Bertacco S, Convertino G, Cuomo A, Donner CF, et al. Inhaled corticosteroids in stable COPD patients: do they have effects on cells and molecular mediators of airway inflammation? Chest 2000;117(6):1633-7. - PubMed
Bensch 2003 {published data only}
-
- Bensch G, Hampel F, Sachs H. Once-daily, evening administration of mometasone furoate dry powder inhaler improves pulmonary function in patients with mild to moderate persistent asthma. European Respiratory Journal 2003;22(Suppl 45):282s.
Boothman‐Burrell 1997 {published data only}
-
- Boothman-Burrell D, Delany SG, Flannery EM, Hancox RJ, Taylor DR. The efficacy of inhaled corticosteroids in the management of non asthmatic chronic airflow obstruction. New Zealand Medical Journal 1997;110(1053):370-3. - PubMed
Brightling 2005 {published data only}
Burge 1999 {published data only}
Chan 1993 {published data only}
-
- Chan CH, Cohen M, Pang J. The effects of inhaled corticosteroids on chronic airflow limitation. Asian Pacific Journal of Allergy and Immunology 1993;11(2):97-101. - PubMed
Confalonieri 1998 {published data only}
Corda 2008 {published data only}
-
- Corda L, Bertella E, La Piana GE, Boni E, Redolfi S, Tantucci C. Inhaled corticosteroids as additional treatment in alpha-1-antitrypsin-deficiency-related COPD. Respiration 2008;76(1):61-8. - PubMed
Cox 1999 {published data only}
-
- Cox G, Whitehead L, Dolovich M, Jordana M, Gauldie J, Newhouse MT. A randomised controlled trial on the effect of inhaled corticosteroids on airways inflammation in adult cigarette smokers. Chest 1999;115(5):1271-7. - PubMed
Culpitt 1999 {published data only}
-
- Culpitt SV, Maziak W, Loukidis S, Nightingale JA, Matthews JL, Barnes PJ. Effect of high dose inhaled steroid on cells, cytokines, and proteases in induced sputum in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1999;160(5 Pt 1):1635-9. - PubMed
Dompeling 1992 {published data only}
-
- Dompeling E, Schayck CP, Molema J, Folgering H, Grunsven PM, Weel C. Inhaled beclomethasone improves the course of asthma and COPD. European Respiratory Journal 1992;5(8):945-52. - PubMed
Dompeling 1993 {published data only}
-
- Dompeling E, Schayck CP, Grunsven PM, Herwaarden CL, Akkermans R, Molema J, et al. Slowing the deterioration of asthma and chronic obstructive pulmonary disease observed during bronchodilator therapy by adding inhaled corticosteroids. Annals of Internal Medicine 1993;118(10):770-8. - PubMed
Egan 1999 {published and unpublished data}
-
- Egan JJ, Maden C, Kalra S, Adams JE, Eastell R, Woodcock AA. A randomised, double blind study comparing the effects of beclomethasone and fluticasone on bone density over 2 years. European Respiratory Journal 1999;13(6):1267-75. - PubMed
Engel 1989 {published data only}
-
- Engel T, Heinig JH, Madsen O, Hansen M, Weeke ER. A trial of inhaled budesonide on airway responsiveness in smokers with chronic bronchitis. European Respiratory Journal 1989;2(10):935-9. - PubMed
Fattore 2005 {published data only}
-
- Fattore G, Torbica A, Mangone M. Cost-analysis of four treatment strategies in the management of moderate-to-severe COPD: an application non-parametric bootstrap. Pharmacoeconomics Italian Research Articles 2005;72(2):135-43.
Fazio 1986 {published data only}
-
- Fazio F, Lafortuna CL. Beclomethasone dipropionate does not affect mucociliary clearance in patients with chronic obstructive lung disease. Respiration 1986;50(1):62-5. - PubMed
Ferreira 2001 {published data only}
-
- Ferreira IM, Hazari MS, Gutierrez C, Zamel N, Chapman KR. Exhaled nitric oxide and hydrogen peroxide in patients with chronic obstructive pulmonary disease: effects of inhaled beclomethasone. American Journal of Respiratory and Critical Care Medicine 2001;164(6):1012-5. - PubMed
Ferreira 2003 {unpublished data only}
-
- Ferreira IM, Sandrini A, Zamel N, Balter M, Chapman KR. Effects of inhaled fluticasone propionate (FP) on Exhaled Nitric Oxide (ENO), functional exercise capacity and quality of life in stable patients with COPD. In: American Thoracic Society 99th International Conference; 2003 May 16-21; Seattle. 2003:B024 Poster 404.
Guenette 2011 {published data only}
-
- Guenette JA, Raghavan N, Harris-McAllister V, Preston ME, Webb KA, O'Donnell DE. Effect of adjunct fluticasone propionate on airway physiology during rest and exercise in COPD. Respiratory Medicine 2011;105(12):1836-45. [1532-3064: (Electronic)] - PubMed
Guleria 2003 {published data only}
-
- Guleria R, Singh TR, Sinha S, Padhy K, Gupta K, Pande JN. Effect of single inhalation of a salbutamol, ipratropium bromide and beclomethasone on mucociliary clearance in patients with chronic obstructive airway disease. Indian Journal of Chest Diseases and Allied Sciences 2003;45(4):241-6. - PubMed
Keatings 1997 {published data only}
-
- Keatings VM, Jatakanon A, Worsdell YM, Barnes PJ. Effects of inhaled and oral glucocorticoids on inflammatory indices in asthma and COPD. American Journal of Respiratory and Critical Care Medicine 1997;155:542-8. - PubMed
Kozak‐Skzopek 1997 {published data only}
-
- Kozak-Skzopek E, Ulmer WT. Inhalative Budesonid-Therapie bei chronischer Bronchitis. Atemweg und Lungenerkrankheiten 1997;23(9):542-6.
Llewellyn‐Jones 1996 {published data only}
-
- Llewellyn-Jones CG, Harris TA, Stockley RA. Effect of fluticasone propionate on sputum of patients with chronic bronchitis and emphysema. American Journal of Respiratory and Critical Care Medicine 1996;153(2):616-21. - PubMed
Loppow 2001 {published data only}
-
- Loppow D, Schleiss MB, Kanniess F, Taube C, Jorres RA, Magnussen H. In patients with chronic bronchitis a four week trial with inhaled steroids does not attenuate airway inflammation. Respiratory Medicine 2001;95(2):115-21. - PubMed
Matlin 1976 {unpublished data only}
-
- Matlin RA. The efficacy of steroid aerosol in chronic obstructive pulmonary disease (COPD). American Review of Respiratory Disease 1976;113(4):184.
Melani 1999 {published data only}
-
- Melani AS, Di Gregorio A. Four-week nebulized beclomethasone dipropionate in stable COPD patients with exertional dyspnoea. Monaldi Archives for Chest Disease 1999;54(3):224-7. - PubMed
Moller 1999 {published data only}
-
- Moller M, Haller S, Kiebart M, Cloes R. AeroBec Autohaler in patients with reversible chronic-obstructive respiratory diseases. Atemwegs- Und Lungenkrankheiten 1999;25(3):160-7.
Nava 2000 {published data only}
-
- Nava S, Compagnoni ML. Controlled short-term trial of fluticasone propionate in ventilator-dependent patients with COPD. Chest 2000;118(4):990-9. - PubMed
NCT00358358 {published data only}
-
- NCT00358358. Chronic Obstructive Pulmonary Disease Endpoints Study [A randomized, double-blind placebo-controlled study of treatments with salmeterol, fluticasone propionate and their combination to evaluate novel endpoints in patients with chronic obstructive pulmonary disease]. www.clinicaltrials.gov/ct2/show/NCT00358358 (first received 31 July 2006).
Nishimura 1999 {published data only}
-
- Nishimura K, Koyama H, Ikeda A, Tsukino M, Hajiro T, Mishima M, et al. The effect of high-dose inhaled beclomethasone dipropionate in patients with stable COPD. Chest 1999;115(1):31-7. - PubMed
Nishimura 2000 {published data only}
-
- Nishimura K, Ikeda A, Koyama H, Zhang M, Tsukino M, Hajiro T, et al. Additive effects of prednisolone and beclomethasone dipropionate in patients with stable chronic obstructive pulmonary disease. Pulmonary Pharmacology and Therapeutics 2000;13(5):225-30. - PubMed
O'Brien 2001 {published data only}
-
- O'Brien A, Russo-Magno P, Karki A, Hiranniramol S, Hardin M, Kaszuba M, et al. Effects of withdrawal of inhaled steroids in men with severe irreversible airflow obstruction. American Journal of Respiratory and Critical Care Medicine 2001;164(3):365-71. - PubMed
Ouyang 1998 {published data only}
-
- Ouyang R, Yin B. Clinical study of inhaled corticosteroid in non-asthmatic chronic obstructive pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi 1998;21(8):497-9. - PubMed
Reid 2008 {published data only}
-
- Reid DW, Wen Y, Johns DP, Williams TJ, Ward C, Walters EH. Bronchodilator reversibility, airway eosinophilia and anti-inflammatory effects of inhaled fluticasone in COPD are not related. Respirology 2008;13:799-809. - PubMed
Robertson 1986 {published data only}
-
- Robertson AS, Gove RI, Wieland GA, Burge PS. A double-blind comparison of oral prednisolone 40 mg/day with inhaled beclomethasone dipropionate 1500 ug/day in patients with adult onset chronic obstructive airways disease. European Journal of Respiratory Diseases 1986;146:565-9. - PubMed
Roth 1996 {published data only}
-
- Roth R. Inhaled corticosteroids are effective and well tolerated [Inhalative Glukokortikoide sind wirksam und gut verträglich]. Therapiewoche 1996;46(24):1380.
Rutgers 1998 {published data only}
-
- Rutgers SR, Koeter GH, Mark TW, Postma DS. Short-term treatment with budesonide does not improve hyperresponsiveness to adenosine 5'-monophosphate in COPD. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Pt 1):880-6. - PubMed
Sandrini 2003 {published data only}
-
- Sandrini A, Plit M, Glaville A, Bryant D, Yates DH. Effect of inhaled steroid withdrawal on exhaled nitric oxide in COPD: preliminary data [Abstract]. Thorax 2003;58(Suppl 3):iii86.
Sapey 2000 {published data only}
-
- Sapey E, Langford NJ, Kendall MJ. Inhaled corticosteroids and chronic obstructive pulmonary disease. Journal of Clinical Pharmacy and Therapeutics 2000;25(4):235-8. - PubMed
Scherr 2012 {published data only}
-
- Scherr A, Schafroth Torok S, Jochmann A, Miedinger D, Maier S, Taegtmeyer AB, et al. Response to add-on inhaled corticosteroids in COPD based on airway hyperresponsiveness to mannitol. Chest 2012;142:919-26. - PubMed
Schuurmans 2001 {published data only}
-
- Schuurmans M, Soler M. COPD – Therapie: Bronchodilatatoren und Steroide. Therapiewoche 2001;17(1):28-32.
Sin 2004 {published data only}
-
- Sin DD, Lacy P, York E, Man SFP. Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2004;170(7):760-5. - PubMed
Sin 2008 {published data only}
-
- Sin DD, Man SF, Marciniuk DD, Ford G, FitzGerald M, Wong E, et al. The effects of fluticasone with or without salmeterol on systemic biomarkers of inflammation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2008;177(11):1207-14. - PubMed
Spicuzza 2004 {published data only}
-
- Spicuzza L, Scuderi V, Prosperini G, Balsamo R, DiMara GU, Polosa R. Acute effect of inhaled fluticasone on airway hyperresponsiveness to adenosine 5'-monophosphate in asthma and in COPD. In: American Thoracic Society 100th International Conference; 2004 May 21-26; Orlando. Vol. A58 Poster K33. 2004.
Thompson 1992 {published data only}
-
- Thompson AB, Mueller MB, Heires AJ, Bohling TL, Daughton D, Yancey SW, et al. Aerosolized beclomethasone in chronic bronchitis. Improved pulmonary function and diminished airway inflammation. American Review of Respiratory Disease 1992;146(2):389-95. [MEDLINE: ] - PubMed
Thompson 2002 {published data only}
-
- Thompson WH, Carvalho P, Souza JP, Charan NB. Controlled trial of inhaled fluticasone propionate in moderate to severe COPD. Lung 2002;180(4):191-201. - PubMed
Tsang 1999 {published data only}
-
- Tsang KW. Inhaled corticosteroids in COPD. Thorax 1999;54(2):186. - PubMed
Turker 2004 {published data only}
-
- Turker H, Karakurt Z, Durucu K, Sulu E, Boga S, Yavsan M. High dose inhaler corticosteroids in patients with COPD. European Respiratory Journal 2004;24(Suppl 48):289s.
van den Boom 2001 {published data only}
-
- den Boom G, Rutten-van Molken MP, Molema J, Tirimanna PR, Weel C, Schayck CP. The cost effectiveness of early treatment with fluticasone propionate 250 mcg twice a day in subjects with obstructive airway disease: results of the DIMCA program. American Journal of Respiratory and Critical Care Medicine 2001;164(11):2057-66. - PubMed
van der Valk 2002 {published data only}
-
- Valk P, Monninkhof E, Phalen J, Zielhuis G, Herwaarden C. Effect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease: the COPE study. American Journal of Respiratory and Critical Care Medicine 2002;166(10):1358-63. - PubMed
van Grunsven 2000 {published data only}
-
- Grunsven PM, Schayck CP, Deuveren M, Herwaarden CL, Akkermans RP, Weel C. Compliance during long-term treatment with fluticasone propionate in subjects with early signs of asthma or chronic obstructive pulmonary disease (COPD): results of the Detection, Intervention, and Monitoring Program of COPD and Asthma (DIMCA) Study. Journal of Asthma 2000;37(3):225-34. - PubMed
van Grunsven 2003 {published data only}
-
- Grunsven P, Schermer T, Akkermans R, Albers M, den Boom G, Schayck O, et al. Short- and long-term efficacy of fluticasone propionate in subjects with early signs and symptoms of chronic obstructive pulmonary disease. Results of the DIMCA study. Respiratory Medicine 2003;97(12):1303-12. - PubMed
van Schayck 1995 {published data only}
-
- Schayck CP, Dompeling E, Rutten MP, Folgering H, den Boom G, Weel C. The influence of an inhaled steroid on quality of life in patients with asthma or COPD. Chest 1995;107(5):1199-205. - PubMed
Verhoeven 2002 {published data only}
Vestbo 2000 {published data only}
-
- Vestbo J, Sørensen T, Lange P, Brix A, Torre P, Viskum K. Long-term effect of inhaled budesonide in patients with mild to moderate chronic obstructive lung disease. The Østerbro Study [Langtidsvirkningen af inhaleret budesonid hos patienter med mild og moderat kronisk obstruktiv lungesygdom. Østerbro lunge studie]. Ugeskrift for Læger 2000;162(4):493-7. - PubMed
Watson 1992 {published data only}
-
- Watson A, Lim TW, Joyce H, Pride NB. Failure of inhaled corticosteroids to modify bronchoconstrictor or bronchodilator responsiveness in middle-aged smokers with mild airflow obstruction. Chest 1992;101(2):350-5. - PubMed
Weiner 1995 {published data only}
-
- Weiner P, Weiner M, Azgad Y, Zamir D. Inhaled budesonide therapy for patients with stable COPD. Chest 1995;108(6):1568-71. - PubMed
Weiner 1997 {published data only}
-
- Weiner P, Zamir D, Beckerman M. Inhaled budesonide for chronic obstructive pulmonary disease. Harefuah 1997;132(11):823. - PubMed
Weiner 1999 {published data only}
-
- Weiner P, Weiner M, Rabner M, Waizman J, Magadle R, Zamir D. The response to inhaled and oral steroids in patients with stable chronic obstructive pulmonary disease. Journal of Internal Medicine 1999;245(1):83-9. - PubMed
Weir 1990 {published data only}
-
- Weir DC, Gove RI, Robertson AS, Burge PS. Response to corticosteroids in chronic airflow obstruction: relationship to emphysema and airways collapse. European Respiratory Journal 1991;4(10):1185-90. - PubMed
Weir 1993 {published data only}
-
- Weir DC, Burge PS. Effects of high dose inhaled beclomethasone dipropionate, 750 mcg and 1500 mcg twice daily, and 40 mg per day oral prednisolone on lung function, symptoms, and bronchial hyperresponsiveness in patients with non-asthmatic chronic airflow obstruction. Thorax 1993;48(4):309-16. - PMC - PubMed
Wempe 1992 {published data only}
Wesseling 1991 {published data only}
-
- Wesseling GJ, Quaedvlieg M, Wouters EF. Inhaled budesonide in chronic bronchitis. Effects on respiratory impedance. European Respiratory Journal 1991;4(9):1101-5. - PubMed
Whittaker 2000 {published data only}
-
- Whittaker AJ, Spiro SG. Inhaled steroid therapy in chronic obstructive pulmonary disease. Current Opinion in Pulmonary Medicine 2000;6(2):104-9. - PubMed
Wilcke 1997 {published data only}
-
- Wilcke JT, Dirksen A. The effect of inhaled glucocorticosteroids in emphysema due to alpha1-antitrypsin deficiency. Respiratory Medicine 1997;91(5):275-9. - PubMed
Williamson 2009 {published data only}
-
- Williamson P, Menzies D, Clearie K, Vaidyanathan S, Lipworth B. Dose response for inhaled fluticasone on airway and systemic inflammation in COPD [Abstract]. In: European Respiratory Society 19th Annual Congress; 2009 Sep 12-15; Vienna. 2009. - PubMed
Yildiz 2000 {published data only}
-
- Yildiz F, Kaur AC, Ilgazli A, Celikoglu M, Kacar Ozkara S, Paksoy N, et al. Inhaled corticosteroids may reduce neutrophilic inflammation in patients with stable chronic obstructive pulmonary disease. Respiration 2000;67(1):71-6. - PubMed
Additional references
Agustí 2019
-
- Agustí A, Hogg JC. Update on the pathogenesis of chronic obstructive pulmonary disease. New England Journal of Medicine 2019;381:1248-56. - PubMed
Burge 2003a
Calverley 2003c
-
- Calverley PM, Spencer S, Willits L, Burge PS, Jones PW. Withdrawal from treatment as an outcome in the ISOLDE study of COPD. Chest 2003;124(4):1350. - PubMed
Celli 2008
-
- Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. American Journal of Respiratory and Critical Care Medicine 2008;178(4):332-8. - PubMed
Celli 2019
-
- Celli BR, Wedzicha JA. Update on clinical aspects of chronic obstructive pulmonary disease. New England Journal of Medicine 2019;381:1257-66. - PubMed
Dabscheck 2022
-
- Dabscheck E, George J, Herrmann K, McDonald CF, McDonald VM, McNamara RJ, O’Brien M, Smith BJ, Zwar NA, Yang IA. COPD-X Australian guidelines for the diagnosis and management of chronic obstructive pulmonary disease: 2022 update. Medical Journal of Australia 2022;217(8):415-23. - PubMed
Drummond 2008
Eichenhorn 2003
-
- Eichenhorn MS, Wise RA, Madhok TC, Gerald LB, Bailey WC, Tashkin DP, et al. Lack of long-term adverse adrenal effects from inhaled triamcinolone: Lung Health Study II. Chest 2003;124(1):57-62. - PubMed
Ferry 2019
Gartlehner 2006
GOLD 2023
-
- Global Initiative for Chronic Obstructive Pulmonary Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2023 report. goldcopd.org/2023-gold-report-2/ (accessed prior to 31 October 2022).
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed January 2022. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Highland 2003
-
- Highland KB, Strange C, Heffner JE. Long-term effects of inhaled corticosteroids on FEV1 in patients with chronic obstructive pulmonary disease: a meta-analysis. Annals of Internal Medicine 2003;138(12):969-73. - PubMed
Johnell 2002
-
- Johnell O, Pauwels R, Löfdahl CG, Laitinen LA, Postma DS, Pride NB, et al. Bone mineral density in patients with chronic obstructive pulmonary disease treated with budesonide Turbuhaler. European Respiratory Journal 2002;19(6):1058-63. - PubMed
Kew 2014
Ko 2016
Miravitlles 2021
Nannini 2013
-
- Nannini LJ, Poole P, Milan SJ, Kesterton A. Combined corticosteroid and long-acting beta2-agonist in one inhaler versus inhaled corticosteroids alone for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No: CD006826. [DOI: 10.1002/14651858.CD006826.pub2] - DOI - PMC - PubMed
RevMan Web 2020 [Computer program]
-
- Review Manager Web (RevMan Web). Version 4.15.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Scanlon 2004
-
- Scanlon PD, Connett JE, Wise RA, Tashkin DP, Madhok T, Skeans M, et al. Loss of bone density with inhaled triamcinolone in Lung Health Study II. American Journal of Respiratory and Critical Care Medicine 2004;170(12):1302-9. - PubMed
Sin 2005
Singh 2009
-
- Singh S, Amin AV, Loke YK. Long-term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: a meta-analysis. Archives of Internal Medicine 2009;169(3):219-29. - PubMed
Soriano 2007
-
- Soriano JB, Sin DD, Zhang X, Anderson JA, Anthonisen NR, Buist AS, et al. A pooled analysis of FEV1 decline in COPD patients randomized to inhaled corticosteroids or placebo. Chest 2007;131:682-9. - PubMed
Sutherland 2003
van Grunsven 1999
Welte 2009
-
- Welte T. Inhaled corticosteroids in COPD and the risk of pneumonia. Lancet 2009;374(9691):668-70. [1474-547X: (Electronic)] - PubMed
Yang 2016
-
- Yang IA, Shaw JG, Goddard JR, Clarke MS, Reid DW. Use of inhaled corticosteroids in COPD: improving efficacy. Expert Review of Respiratory Medicine 2016;10(3):339-50. - PubMed
Yang 2019
-
- Yang M, Du Y, Chen H, Jiang D, Xu Z. Inhaled corticosteroids and risk of pneumonia in patients with chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials. International Immunopharmacology 2019;77:105950. [DOI: ] - PubMed
Yang 2022
-
- Yang IA, Jenkins CR, Salvi S. COPD in never smokers: pathogenesis and implications for prevention and treatment. Lancet Respiratory Medicine 2022;10(5):497-511. - PubMed
References to other published versions of this review
Yang 2001
Yang 2007
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

