Mitochondrial pyruvate dehydrogenase kinases contribute to platelet function and thrombosis in mice by regulating aerobic glycolysis
- PMID: 36971790
- PMCID: PMC10230171
- DOI: 10.1182/bloodadvances.2023010100
Mitochondrial pyruvate dehydrogenase kinases contribute to platelet function and thrombosis in mice by regulating aerobic glycolysis
Abstract
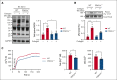
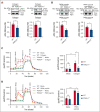
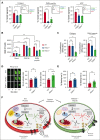
Resting platelets rely on oxidative phosphorylation (OXPHOS) and aerobic glycolysis (conversion of glucose to lactate in the presence of oxygen) for their energy requirements. In contrast, platelet activation exhibits an increased rate of aerobic glycolysis relative to OXPHOS. Mitochondrial enzymes pyruvate dehydrogenase kinases (PDKs) phosphorylate the pyruvate dehydrogenase (PDH) complex to inhibit its activity, thereby diverting the pyruvate flux from OXPHOS to aerobic glycolysis upon platelet activation. Of 4 PDK isoforms, PDK2 and PDK4 (PDK2/4) are predominantly associated with metabolic diseases. Herein, we report that the combined deletion of PDK2/4 inhibits agonist-induced platelet functions, including aggregation, integrin αIIbβ3 activation, degranulation, spreading, and clot retraction. In addition, collagen-mediated PLCγ2 phosphorylation and calcium mobilization were significantly reduced in PDK2/4-/- platelets, suggesting impaired GPVI signaling. The PDK2/4-/- mice were less susceptible to FeCl3-induced carotid and laser-induced mesenteric artery thrombosis without any effect on hemostasis. In adoptive transfer experiments, thrombocytopenic hIL-4Rα/GPIbα-transgenic mice transfused with PDK2/4-/- platelets exhibited less susceptibility to FeCl3 injury-induced carotid thrombosis compared with hIL-4Rα/GPIbα-Tg mice transfused with WT platelets, suggesting a platelet-specific role of PDK2/4 in thrombosis. Mechanistically, the inhibitory effects of PDK2/4 deletion on platelet function were associated with reduced PDH phosphorylation and glycoPER in activated platelets, suggesting that PDK2/4 regulates aerobic glycolysis. Finally, using PDK2 or PDK4 single KO mice, we identified that PDK4 plays a more prominent role in regulating platelet secretion and thrombosis compared with PDK2. This study identifies the fundamental role of PDK2/4 in regulating platelet functions and identifies the PDK/PDH axis as a potentially novel antithrombotic target.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures





References
-
- Mazumdar C, Driggers EM, Turka LA. The untapped opportunity and challenge of immunometabolism: a new paradigm for drug discovery. Cell Metab. 2020;31(1):26–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

