Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer
- PMID: 36972022
- PMCID: PMC10351614
- DOI: 10.1056/NEJMoa2302312
Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer
Abstract
Background: Standard first-line chemotherapy for endometrial cancer is paclitaxel plus carboplatin. The benefit of adding pembrolizumab to chemotherapy remains unclear.
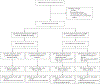
Methods: In this double-blind, placebo-controlled, randomized, phase 3 trial, we assigned 816 patients with measurable disease (stage III or IVA) or stage IVB or recurrent endometrial cancer in a 1:1 ratio to receive pembrolizumab or placebo along with combination therapy with paclitaxel plus carboplatin. The administration of pembrolizumab or placebo was planned in 6 cycles every 3 weeks, followed by up to 14 maintenance cycles every 6 weeks. The patients were stratified into two cohorts according to whether they had mismatch repair-deficient (dMMR) or mismatch repair-proficient (pMMR) disease. Previous adjuvant chemotherapy was permitted if the treatment-free interval was at least 12 months. The primary outcome was progression-free survival in the two cohorts. Interim analyses were scheduled to be triggered after the occurrence of at least 84 events of death or progression in the dMMR cohort and at least 196 events in the pMMR cohort.
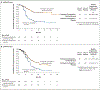
Results: In the 12-month analysis, Kaplan-Meier estimates of progression-free survival in the dMMR cohort were 74% in the pembrolizumab group and 38% in the placebo group (hazard ratio for progression or death, 0.30; 95% confidence interval [CI], 0.19 to 0.48; P<0.001), a 70% difference in relative risk. In the pMMR cohort, median progression-free survival was 13.1 months with pembrolizumab and 8.7 months with placebo (hazard ratio, 0.54; 95% CI, 0.41 to 0.71; P<0.001). Adverse events were as expected for pembrolizumab and combination chemotherapy.
Conclusions: In patients with advanced or recurrent endometrial cancer, the addition of pembrolizumab to standard chemotherapy resulted in significantly longer progression-free survival than with chemotherapy alone. (Funded by the National Cancer Institute and others; NRG-GY018 ClinicalTrials.gov number, NCT03914612.).
Copyright © 2023 Massachusetts Medical Society.
Figures
Comment in
-
Adding immune-checkpoint inhibitors to chemotherapy extends survival in endometrial cancer.Nat Rev Clin Oncol. 2023 Jun;20(6):353. doi: 10.1038/s41571-023-00763-0. Nat Rev Clin Oncol. 2023. PMID: 37024681 No abstract available.
-
PD-1 and PD-L1 Blockade plus Chemotherapy in Endometrial Cancer.N Engl J Med. 2023 Aug 31;389(9):866. doi: 10.1056/NEJMc2308037. N Engl J Med. 2023. PMID: 37646693 No abstract available.
-
PD-1 and PD-L1 Blockade plus Chemotherapy in Endometrial Cancer.N Engl J Med. 2023 Aug 31;389(9):866-867. doi: 10.1056/NEJMc2308037. N Engl J Med. 2023. PMID: 37646694 No abstract available.
-
PD-1 and PD-L1 Blockade plus Chemotherapy in Endometrial Cancer. Reply.N Engl J Med. 2023 Aug 31;389(9):867. doi: 10.1056/NEJMc2308037. N Engl J Med. 2023. PMID: 37646695 No abstract available.
-
PD-1 and PD-L1 Blockade plus Chemotherapy in Endometrial Cancer. Reply.N Engl J Med. 2023 Aug 31;389(9):867-868. doi: 10.1056/NEJMc2308037. N Engl J Med. 2023. PMID: 37646696 No abstract available.
-
Adding immunotherapy to chemotherapy improves survival for endometrial cancer patients.CA Cancer J Clin. 2023 Sep-Oct;73(5):445-447. doi: 10.3322/caac.21809. CA Cancer J Clin. 2023. PMID: 37665330 No abstract available.
-
Immune checkpoint inhibitors: here to stay.Transl Cancer Res. 2023 Oct 31;12(10):2438-2441. doi: 10.21037/tcr-23-1343. Epub 2023 Oct 20. Transl Cancer Res. 2023. PMID: 37969375 Free PMC article. No abstract available.
References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17–48. - PubMed
-
- McAlpine JN, Temkin SM, Mackay HJ. Endometrial cancer: not your grandmother’s cancer. Cancer 2016;122:2787–98. - PubMed
-
- Wan YL, Beverley-Stevenson R, Carlisle D, et al. Working together to shape the endometrial cancer research agenda: the top ten unanswered research questions. Gynecol Oncol 2016;143:287–93. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 CA180868/CA/NCI NIH HHS/United States
- UG1 CA189971/CA/NCI NIH HHS/United States
- P30 CA086862/CA/NCI NIH HHS/United States
- P30CA008748/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U10CA180868/CA/NCI NIH HHS/United States
- U10CA180822/CA/NCI NIH HHS/United States
- U10 CA180863/CA/NCI NIH HHS/United States
- UG1 CA233191/CA/NCI NIH HHS/United States
- P30 CA016058/CA/NCI NIH HHS/United States
- U10 CA180822/CA/NCI NIH HHS/United States
- UG1 CA233339/CA/NCI NIH HHS/United States
- UG1 CA233290/CA/NCI NIH HHS/United States
- UG1 CA233331/CA/NCI NIH HHS/United States
- UG1 CA233330/CA/NCI NIH HHS/United States
- P30 ES001247/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
