Versatile Diphosphine Chelators for Radiolabeling Peptides with 99mTc and 64Cu
- PMID: 36972174
- PMCID: PMC10731650
- DOI: 10.1021/acs.inorgchem.3c00426
Versatile Diphosphine Chelators for Radiolabeling Peptides with 99mTc and 64Cu
Abstract
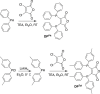
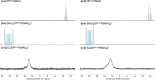
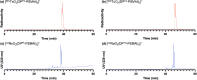
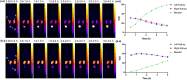
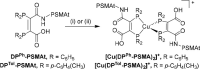
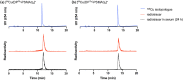
We have developed a diphosphine (DP) platform for radiolabeling peptides with 99mTc and 64Cu for molecular SPECT and PET imaging, respectively. Two diphosphines, 2,3-bis(diphenylphosphino)maleic anhydride (DPPh) and 2,3-bis(di-p-tolylphosphino)maleic anhydride (DPTol), were each reacted with a Prostate Specific Membrane Antigen-targeted dipeptide (PSMAt) to yield the bioconjugates DPPh-PSMAt and DPTol-PSMAt, as well as an integrin-targeted cyclic peptide, RGD, to yield the bioconjugates DPPh-RGD and DPTol-RGD. Each of these DP-PSMAt conjugates formed geometric cis/trans-[MO2(DPX-PSMAt)2]+ (M = 99mTc, 99gTc, natRe; X = Ph, Tol) complexes when reacted with [MO2]+ motifs. Furthermore, both DPPh-PSMAt and DPTol-PSMAt could be formulated into kits containing reducing agent and buffer components, enabling preparation of the new radiotracers cis/trans-[99mTcO2(DPPh-PSMAt)2]+ and cis/trans-[99mTcO2(DPTol-PSMAt)2]+ from aqueous 99mTcO4- in 81% and 88% radiochemical yield (RCY), respectively, in 5 min at 100 °C. The consistently higher RCYs observed for cis/trans-[99mTcO2(DPTol-PSMAt)2]+ are attributed to the increased reactivity of DPTol-PSMAt over DPPh-PSMAt. Both cis/trans-[99mTcO2(DPPh-PSMAt)2]+ and cis/trans-[99mTcO2(DPTol-PSMAt)2]+ exhibited high metabolic stability, and in vivo SPECT imaging in healthy mice revealed that both new radiotracers cleared rapidly from circulation, via a renal pathway. These new diphosphine bioconjugates also furnished [64Cu(DPX-PSMAt)2]+ (X = Ph, Tol) complexes rapidly, in a high RCY (>95%), under mild conditions. In summary, the new DP platform is versatile: it enables straightforward functionalization of targeting peptides with a diphosphine chelator, and the resulting bioconjugates can be simply radiolabeled with both the SPECT and PET radionuclides, 99mTc and 64Cu, in high RCYs. Furthermore, the DP platform is amenable to derivatization to either increase the chelator reactivity with metallic radioisotopes or, alternatively, modify the radiotracer hydrophilicity. Functionalized diphosphine chelators thus have the potential to provide access to new molecular radiotracers for receptor-targeted imaging.
Conflict of interest statement
The authors declare the following competing financial interest(s): A PCT application describing chemical technology included in this manuscript has recently been filed.
Figures

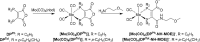



References
-
- Maurer T.; Robu S.; Schottelius M.; Schwamborn K.; Rauscher I.; van den Berg N. S.; van Leeuwen F. W. B.; Haller B.; Horn T.; Heck M. M.; Gschwend J. E.; Schwaiger M.; Wester H. J.; Eiber M. 99mTechnetium-Based Prostate-Specific Membrane Antigen–Radioguided Surgery in Recurrent Prostate Cancer. Eur. Urol. 2019, 75, 659–666. 10.1016/j.eururo.2018.03.013. - DOI - PubMed
-
- Schmidkonz C.; Hollweg C.; Beck M.; Reinfelder J.; Goetz T. I.; Sanders J. C.; Schmidt D.; Prante O.; Bäuerle T.; Cavallaro A.; Uder M.; Wullich B.; Goebell P.; Kuwert T.; Ritt P. 99mTc-MIP-1404-SPECT/CT for the Detection of PSMA-Positive Lesions in 225 Patients with Biochemical Recurrence of Prostate Cancer. Prostate 2018, 78, 54–63. 10.1002/pros.23444. - DOI - PubMed
-
- Hicks R. J.; Jackson P.; Kong G.; Ware R. E.; Hofman M. S.; Pattison D. A.; Akhurst T.; Drummond E.; Roselt P.; Callahan J.; Price R.; Jeffery C. M.; Hong E.; Noonan W.; Herschtal A.; Hicks L. J.; Hedt A.; Harris M.; Paterson B. M.; Donnelly P. S. First-in-Human Trial of 64Cu-SARTATE PET Imaging of Patients with Neuroendocrine Tumours Demonstrates High Tumor Uptake and Retention, Potentially Allowing Prospective Dosimetry for Peptide Receptor Radionuclide Therapy. J. Nucl. Med. 2019, 60, 777–785. 10.2967/jnumed.118.217745. - DOI - PMC - PubMed
-
- Rivas C.; Jackson J. A.; Hungnes I. N.; Ma M. T.. Radioactive Metals in Imaging and Therapy. Comprehensive Coordination Chemistry III; Elsevier, 2021; Vol. 9, pp 706–740.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

